Switch to DRV/r monotherapy
MONOI Study: Switch to DRV/r bid monotherapy
Original article : AIDS. 2010 Sep 24;24(15):2365-74 – C Katlama ;
Marcelin AG, CROI 2011; Abs.533
Last update :
28/03/2014
Dr Anton Pozniak
Chelsea and Westminster Hospital
London, UK

- From W48 results
- Because of the discordance between per-protocol and ITT analysis, DRV/r bid monotherapy was not non inferior to DRV/r bid + 2 NRTI, in patients with virologic suppression on prior ARV regimen
- Sub-group analysis showed that the difference in efficacy favouring the triple arm therapy was larger in patients with a high level of pre-therapy HIV-1 RNA
- A higher proportion of intermittent viremia was seen in patients randomised to DRV/r monotherapy, but no DRV resistance mutations emerged
- There were 2 patients with neurological symptoms and discordant CSF-plasma HIV-1 RNA levels

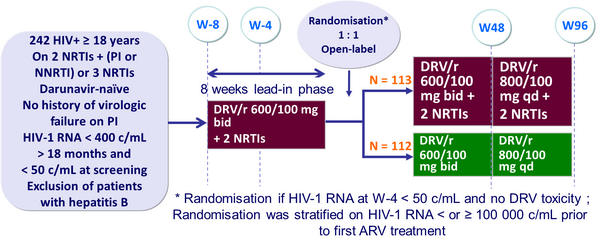
Design :
Objective :
- Non inferiority in the proportion of patients with treatment success at W48 (per-protocol and ITT analysis) ; lower limit of the two-sided 90% CI for the difference = -10%, 80% power
- Treatment failure: virologic failure (2 consecutive HIV-1 RNA > 400 c/mL or 1 value > 400 c/mL and a missing confirmation), treatment modification or discontinuation, withdrawal
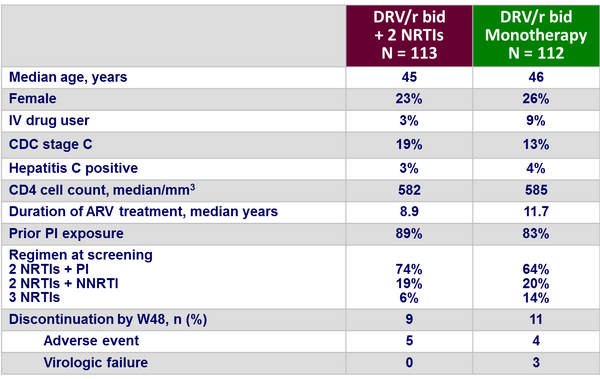
Baseline characteristics and patient disposition :
Primary endpoint: Therapeutic success (HIV-1 RNA < 400 c/mL) at W48 :
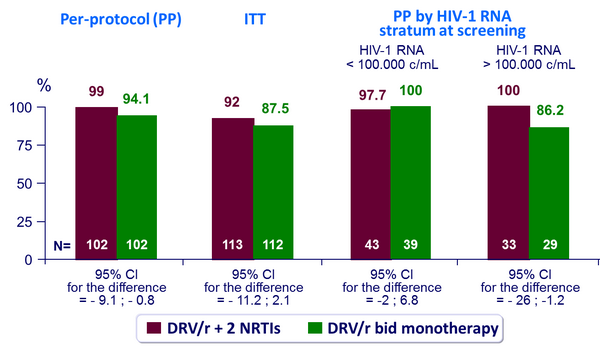
- Non inferiority of DRV/r monotherapy not demonstrated
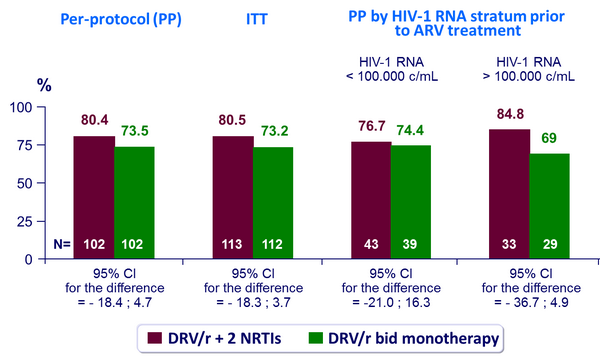
HIV-1 RNA < 50 c/mL at W48 :
Other outcomes at W48 :
- Virologic failure
- 3 in the DRV/r monotherapy arm vs none in the triple therapy arm
- 1 patient with V11I mutation (already present before baseline)
- Resistance
- No DRV resistance mutations in the 13 patients with 2 consecutive�HIV-1 RNA > 50 c/mL (11 in the monotherapy group and 2 in the triple therapy group)
- CD4 counts
- No difference in median increase between groups
- Adherence
- Adherence was associated with virologic success
- Baseline HIV-RNA between 50 and 400 c/mL was associated�with subsequent HIV-1 RNA > 50 c/mL
- 2 patients with neurologic symptoms had HIV-1 RNA > 50 c/mL�in the CSF(330 ; 580), while < 50 c/mL in the plasma
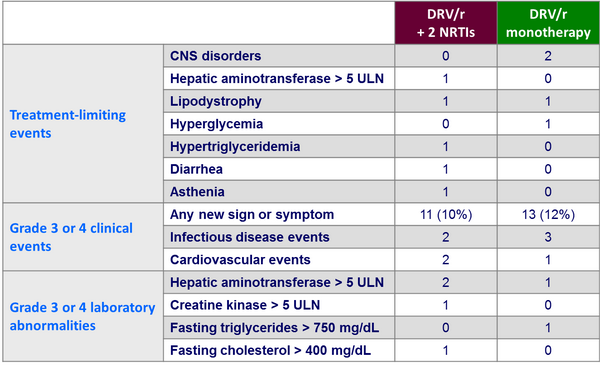
Adverse events :
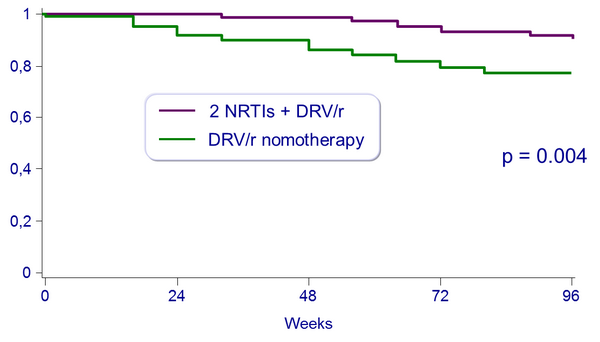
Proportion of patients free of virologic rebound (2 consecutive HIV-1 RNA > 50 c/mL) :
 Back to Table of Contents
Back to Table of Contents



