Van Wyk J. Clin Infect Dis 2020 Jan 6 (Epub ahead of print]
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG + 3TC
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG + 3TC
- Switching to DTG/3TC FDC was non-inferior to remaining on a TAF/FTC-based regimen through Week 48 in ART-experienced, virologically suppressed adults
- No confirmed virologic withdrawals in the DTG/3TC group
- Zero resistance development in the DTG/3TC group
- Good safety profile of DTG/3TC FDC
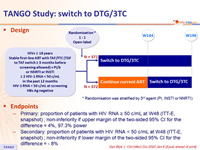
Design
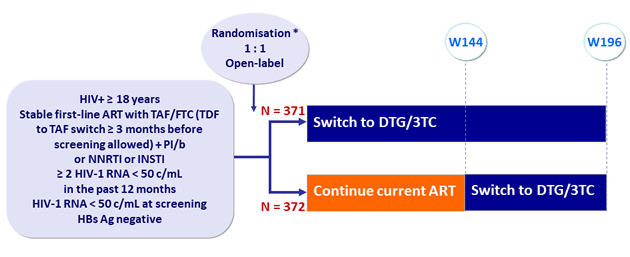
* Randomisation was stratified by 3rd agent (PI, INSTI or NNRTI)
Endpoints
- Primary: proportion of patients with HIV RNA ≥ 50 c/mL at W48 (ITT-E, snapshot) ; non-inferiority if upper margin of the two-sided 95% CI for the difference = 4%, 97.3% power
- Secondary: proportion of patients with HIV RNA < 50 c/mL at W48 (ITT-E, snapshot) ; non-inferiority if lower margin of the two-sided 95% CI for the difference = - 8%
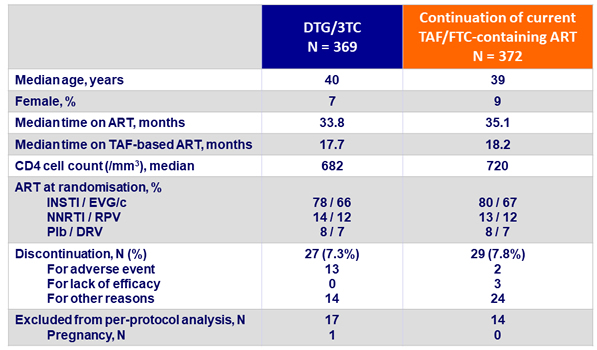
Baseline characteristics and patient disposition
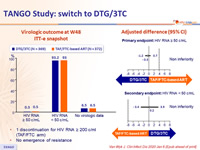
Virologic outcome at W48 ITT-e snapshot
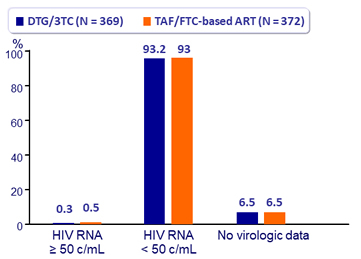
- 1 discontinuation for HIV RNA ≥ 200 c/ml (TAF/FTC arm)
- No emergence of resistance
Adjusted difference (95% CI)
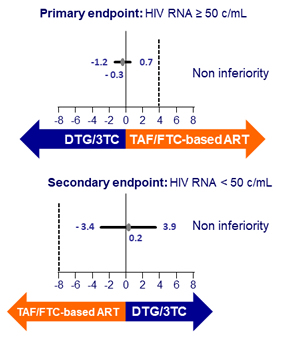
Other efficacy results
- HIV RNA ≥ 50 c/mL at W48, per-protocol population
- DTG/3TC = 0 vs TAF-based ART = 0.6% (difference [95% CI] : -0.6 % [- 1.3 to 0.2])
- Post-hoc analysis on baseline proviral DNA genotyping
- Pre-existing archived M184V/I : 4/322 DTG/3TC and 3/321 TAF-based ART
- HIV RNA < 50 c/mL at W48 in 7/7
- Median CD4/mm3 increase at W48
- DTG/3TC = + 22.5 vs TAF-based ART = + 11
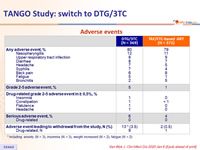
Adverse events
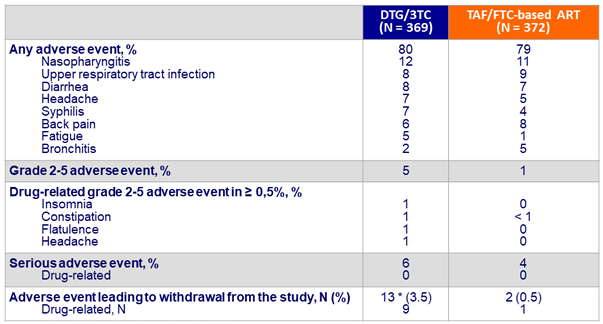
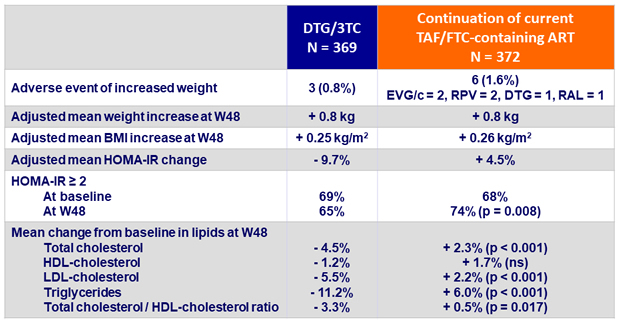
Weight increase and metabolic parameters
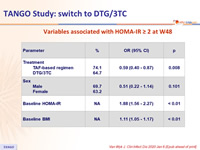
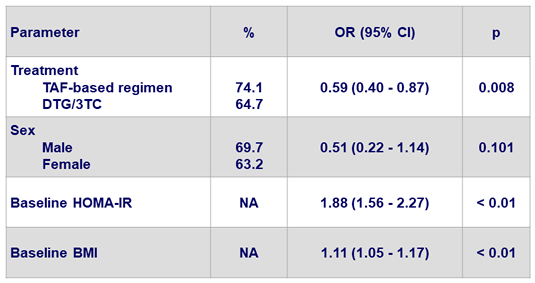
Variables associated with HOMA-IR ≥ 2 at W48
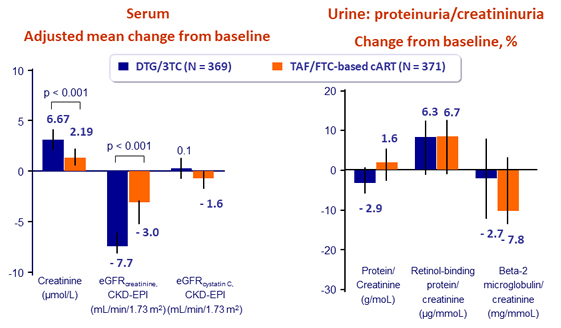
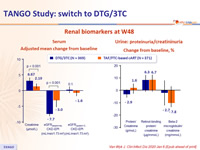
Renal biomarkers at W48