Nguyen A. AIDS. 2011 Jul 31;25(12):1481-7
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to RAL-containing regimen
» RAL + 2 NRTI vs EFV + 2 NRTI
Switch studies in virologically suppressed patients
» Switch to RAL-containing regimen
» RAL + 2 NRTI vs EFV + 2 NRTI
Drugs
RAL, EFV, 2 NRTI
RAL, EFV, 2 NRTI
- Half of patients previously on a stable EFV preferred to switch to RAL, after double -blind exposure to RAL for 2 weeks
- Substitution of EFV by RAL significantly impacted on lipid levels, stress, and anxiety scores
- After study completion, 51 % of patients switched to RAL
- Study limitations
- Small sample size
- Few women
- Exclusion of patients not tolerating EFV
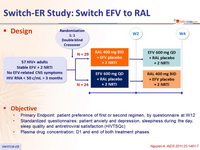
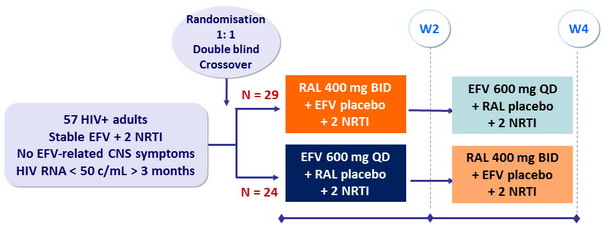
Design
Objective
- Primary Endpoint: patient preference of first or second regimen, by questionnaire at W12
- Standardized questionnaires: patient anxiety and depression, sleepiness during the day, sleep quality and antiretroviral satisfaction ( HIVTSQc )
- Plasma drug concentration: D1 and end of both treatment phases
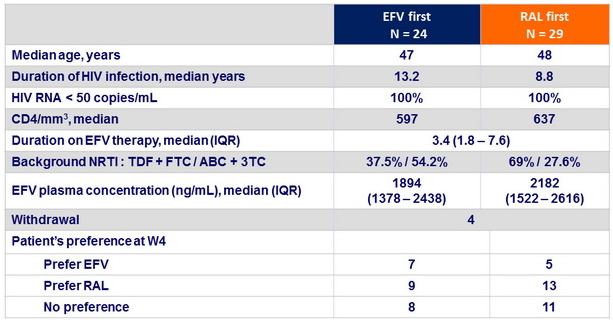
Baseline characteristics and outcome
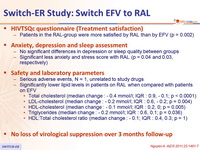
HIVTSQc questionnaire (Treatment satisfaction)
- Patients in the RAL-group were more satisfied by RAL than by EFV (p = 0.002)
Anxiety, depression and sleep assessment
- No significant differences in depression or sleep quality between groups
- Significant less anxiety and stress score with RAL (p = 0.04 and 0.03, respectively)
Safety and laboratory parameters
- Serious adverse events, N = 1, unrelated to study drugs
- Significantly lower lipid levels in patients on RAL when compared with patients on EFV
- Total cholesterol (median change : - 0.4 mmol /l; IQR : 0.9, - 0.1; p < 0.0001)
- LDL-cholesterol (median change : - 0.2 mmol /l; IQR : 0.6, - 0.2; p = 0.004)
- HDL-cholesterol ( median change : - 0.1 mmol /l; IQR : 0.2, 0; p = 0.005)
- Triglycerides ( median change : - 0.2 mmol /l ; IQR : 0.6, 0.1; p = 0.036)
- HDL:Total cholesterol ratio (median change : - 0.1; IQR : 0.4, 0.3; p = 1 )