Campo R. Clin Infect Dis. 2013 Jun;56(11):1637-45
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch ABC/3TC to TDF/FTC
» ABC/3TC vs FTC/TDF
Switch studies in virologically suppressed patients
» Switch ABC/3TC to TDF/FTC
» ABC/3TC vs FTC/TDF
Drugs
FTC/TDF, ABC/3TC, TDF, ABC, FTC, 3TC
FTC/TDF, ABC/3TC, TDF, ABC, FTC, 3TC
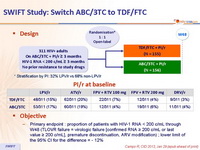
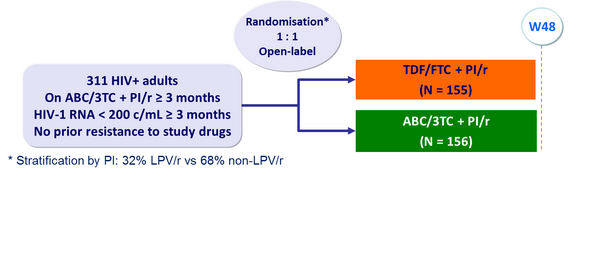
Design :
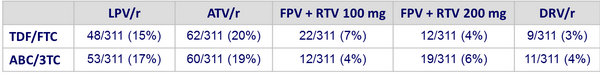
PI/r at baseline :
Objective :
- Primary endpoint : proportion of patients with HIV-1 RNA < 200 c/mL through W48 (TLOVR failure = virologic failure [confirmed RNA ≥ 200 c/mL or last value ≥ 200 c/mL], premature discontinuation, ARV modification) ; lower limit of the 95% CI for the difference = - 12%
Baseline characteristics and patient disposition :
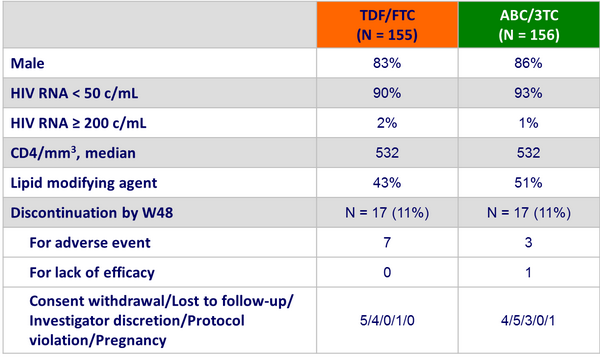
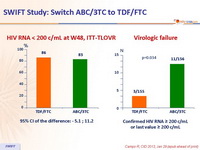
HIV RNA < 200 c/mL at W48, ITT-TLOVR
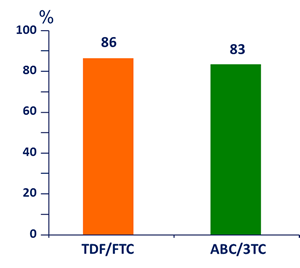
95% CI of the difference: - 5.1 ; 11.2
Virologic failure
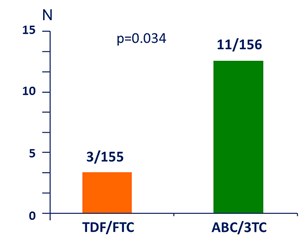
Confirmed HIV RNA ≥ 200 c/mL or last value ≥ 200Â c/mL
Adverse event leading to study drug discontinuation :
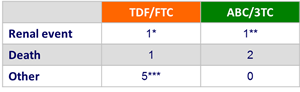
* Creatinine elevation
** Renal failure/dehydration
*** Multiple CNS symptoms and rash ; malaise and lower back pain ; sepsis ; rash ; decreased weight
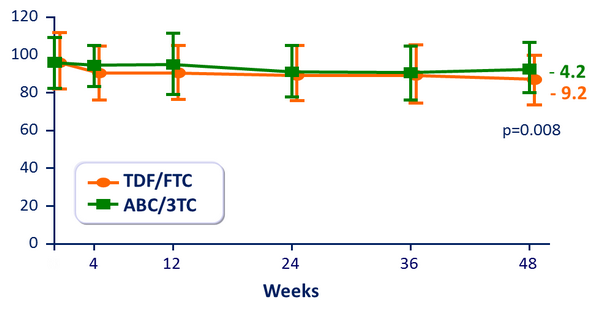
eGFR (MDRD) (mL/min/1.73 m2) :
Fasting Lipids: median change from baseline to W48, mg/dL [mmol/L] :
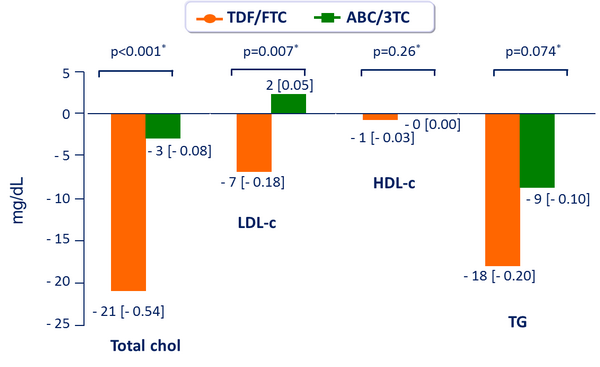
- No significant difference between groups in total cholesterol/HDL-c ratio at W48
* Wilcoxon rank-sum test