Karlström O. J Acquir Immune Defic Syndr. 2007 Apr 1;44(4):417-22
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
Drugs
ATV/r
ATV/r
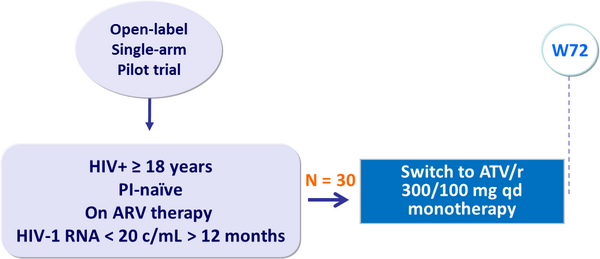
- Failure of ATV/r as maintenance monotherapy
Design :
Primary endpoint :
- Absence of virologic failure by W72 (2 consecutive HIV-1 RNA > 20 c/mL)
- 15 patients enrolled
- Prior ARV therapy

- Median CD4 cell count/mm3: at inclusion = 440 ; nadir = 200
- Median HIV-1 RNA before initiation of ARV therapy: 5.1 log10 c/mL
- Prior ARV therapy
- 5 virologic failures -> termination of the study
- No PI resistance in samples from patients with virologic failure



