Daar ES. Lancet 2018;5:e347-56
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to BIC/FTC/TAF
» BIC/FTC/TAF vs ATV/r or ATV/c or DRV/r or DRV/c + ABC/3TC or FTC/TDF
Switch studies in virologically suppressed patients
» Switch to BIC/FTC/TAF
» BIC/FTC/TAF vs ATV/r or ATV/c or DRV/r or DRV/c + ABC/3TC or FTC/TDF
Drugs
BIC/FTC/TAF, DRV/c, DRV/r, ATV/r, FTC/TAF, FTC/TDF, ABC/3TC, TAF, TDF, ABC, FTC, 3TC
BIC/FTC/TAF, DRV/c, DRV/r, ATV/r, FTC/TAF, FTC/TDF, ABC/3TC, TAF, TDF, ABC, FTC, 3TC
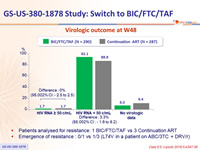
- Switching to BIC/FTC/TAF was non-inferior to remaining on a boosted protease inhibitor + 2 NRTI
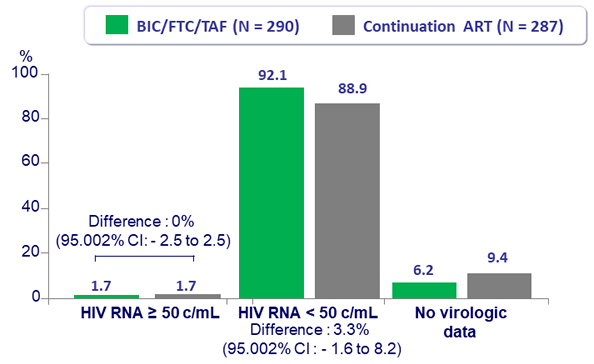
- 1.7% of subjects in each arm had HIV-1 RNA ≥ 50 c/mL through 48 weeks
- 92.1% of subjects treated with BIC/FTC/TAF maintained virologic suppression vs 88.9% in the continuation arm
- No treatment emergent resistance in patients who switched to BIC/FTC/TAF
- 1 subject who continued DRV/r + ABC/3TC developed resistance mutation to ABC
- BIC/FTC/TAF was well tolerated
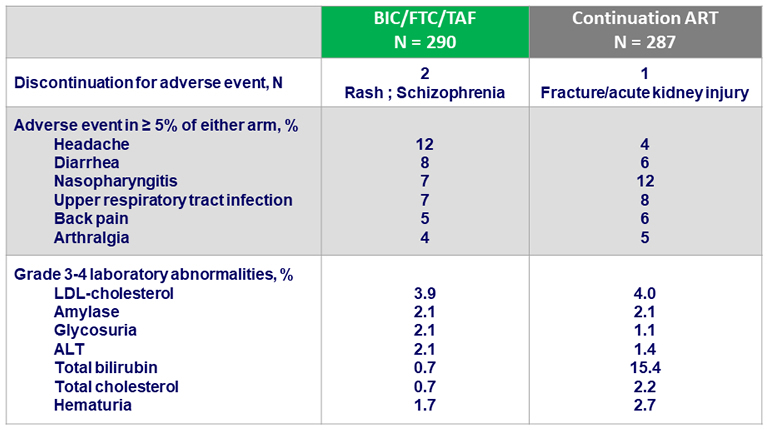
- Adverse events were comparable between arms at week 48
- mild headache was reported more with BIC/FTC/TAF but was mostly transient and low grade
- Less than 1% of patients discontinued due to an adverse event in both arms
- No difference in grade 3 or 4 laboratory abnormalities between arms, except for more total bilirubin abnormalities in continuation arm due to ATV use
- Statistically significant improvements in triglycerides and total cholesterol:HDL ratio in subjects who switched to BIC/FTC/TAF
- Adverse events were comparable between arms at week 48
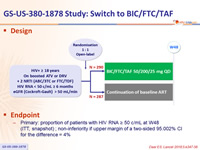
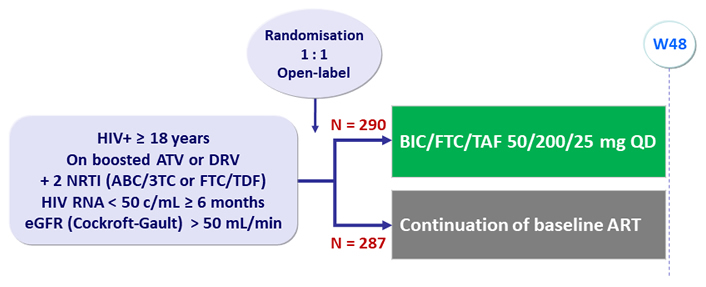
Design
Endpoint
- Primary: proportion of patients with HIV RNA ≥ 50 c/mL at W48 (ITT, snapshot) ; non-inferiority if upper margin of a two-sided 95.002% CI for the difference = 4%
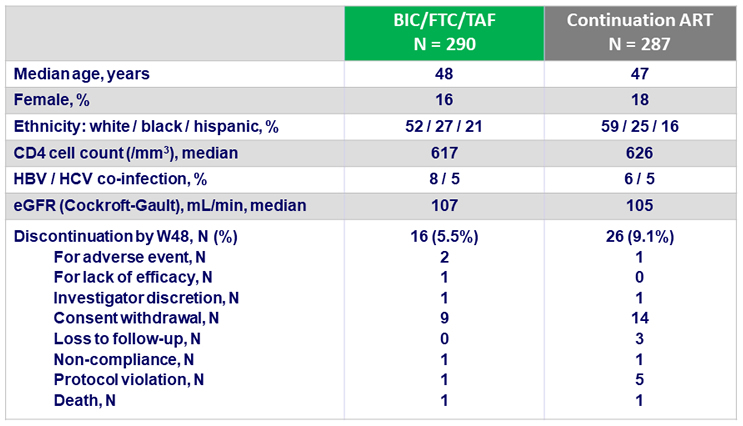
Baseline characteristics and patient disposition
Virologic outcome at W48
- Patients analysed for resistance: 1 BIC/FTC/TAF vs 3 Continuation ART
- Emergence of resistance : 0/1 vs 1/3 (L74V in a patient on ABC/3TC + DRV/r)
Adverse events between D0 and W48, %
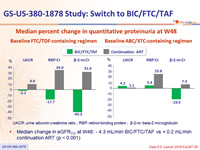
Median percent change in quantitative proteinuria at W48
Baseline
FTV/TDF-containing regimen (left) ABC/3TC-containing regimen (right)
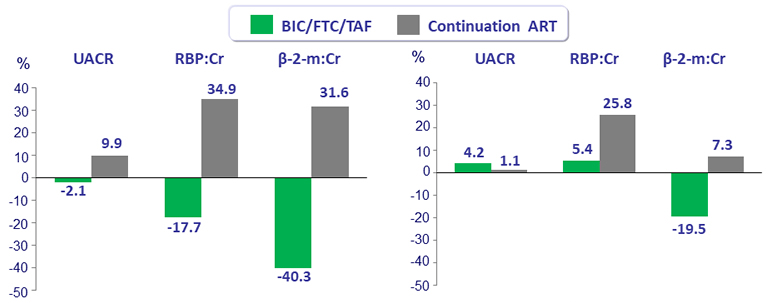
UACR: urine albumin:creatinine ratio ; RBP: retinol-binding protein ; β-2- m: beta-2 microglobulin
- Median change in eGFRCG at W48:
- 4.3 mL/min BIC/FTC/TAF vs + 0.2 mL/min continuation ART (p < 0.001)
Median Fasting Lipid Changes at Week 48 (mg/dL)
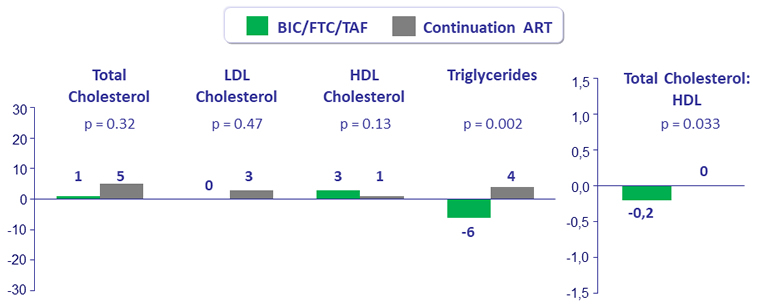
- Taking lipid lowering agents at baseline: B/F/TAF : 16.2%, Continuation ART : 15.7%, p = 0.91
- Initiated lipid lowering agents during the study: B/F/TAF : 2.8%, Continuation ART: 3.5%, p = 0.64