Orkin C. Lancet HIV 2017 ; 4:e195-204
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch TDF to TAF
» RPV/FTC/TDF vs RPV/FTC/TAF
Switch studies in virologically suppressed patients
» Switch TDF to TAF
» RPV/FTC/TDF vs RPV/FTC/TAF
Drugs
RPV, FTC/TAF, FTC/TDF, TAF, TDF, FTC
RPV, FTC/TAF, FTC/TDF, TAF, TDF, FTC
- Overall, virally suppressed, HIV-1 infected participants who switched to rilpivirine, emtricitabine, and tenofovir alafenamide maintained viral suppression at 48 weeks with low rates of virological failure, good tolerability, and improvements in measures of bone and renal safety compared with rilpivirine, emtricitabine, and tenofovir disoproxil fumarate
Design
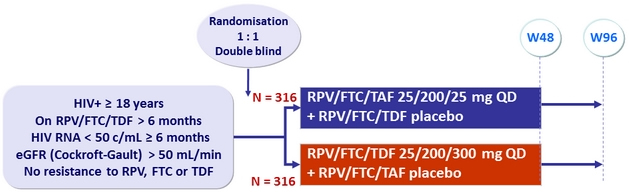
Endpoints
- Primary: proportion of patients maintaining HIV RNA < 50 c/mL at W48 (ITT, snapshot) ; non-inferiority if lower margin of a two-sided 95.001% CI for the difference = - 8%, 85% power
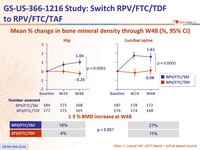
- Secondary: percentage change for hip and spine bone mineral density between treatment groups ; 90% power to detect a 1.38% difference (non-inferiority margin) ; multiple adjustments to test for superiority
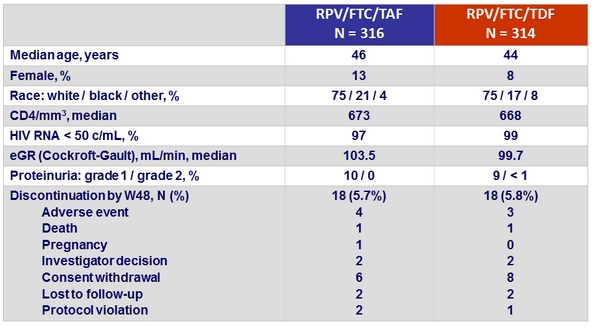
Baseline characteristics and outcome
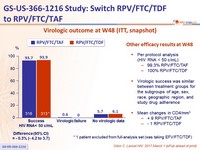
Virologic outcome at W48 (ITT, snapshot)
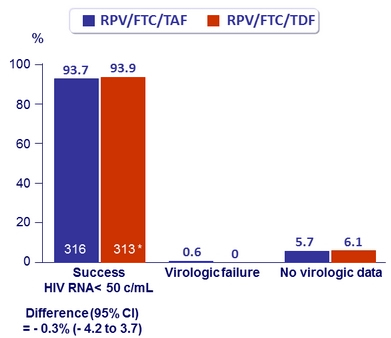
* 1 patient excluded from full- analysis set ( was taking EFV/FTC/TDF)
Other efficacy results at W48
- Per protocol analysis
(HIV RNA < 50 c/mL)
- ‒ 99.3% RPV/FTC/TAF ‒
- 100% RPV/FTC/TDF
- Virologic success was similar between treatment groups for the subgroups of age, sex, race, geographic region, and study drug adherence
- Mean changes in CD4/mm3
- ‒ + 9 RPV/FTC/TAF
- - 1 RPV/FTC/TDF
Resistance analysis
- Genotype and Phenotype testing if confirmed HIV RNA ≥ 50 c/mL
and confirmatory sample ≥ 400 c/mL, or HIV RNA ≥ 400 c/mL at W48
or at the last visit on study drug
- 1 patient in the RPV/FTC/TAF group: re-emergence of archived mutations M41K, E44D, D67N, V118I, L210W, T215Y; no new mutations (did not re-suppress)
- 1 patient in the RPV/FTC/TDF group: no resistance detected, was re-suppressed on continued therapy
- Historical genotypes: resistance mutations to study drug in 3 participants
- 3 patients in the TAF group: M184V (N = 2), E138A, K101E + E138K
- 1 discontinued at W4 with HIV RNA < 50 c/mL, 3 with HIV RNA < 50 c/mL at W48
- 3 patients in the TDF group: M184V, E138A (N = 2)
- All 3 with HIV RNA < 50 c/mL at W48
- 3 patients in the TAF group: M184V (N = 2), E138A, K101E + E138K
Adverse events, %
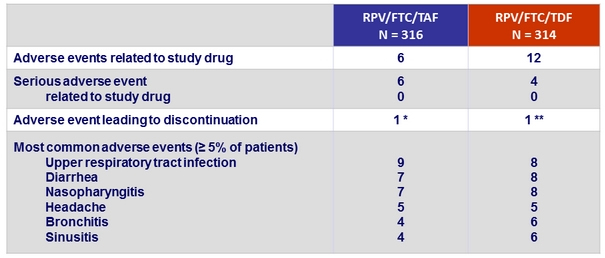
* Gastroesophageal reflux disease (N = 1), hiatus hernia and ulcerative oesophagitis (N = 1), fatigue
(N = 1), leading to discontinuation), suicidal depression (N = 1)
** Drug hypersensitivity (N = 1), leading to discontinuation), increased ALT and AST (N = 1), chronic myeloid leukaemia (N = 1)
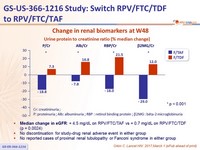
Change in renal biomarkers at W48
Urine protein to creatinine ratio (% median change)
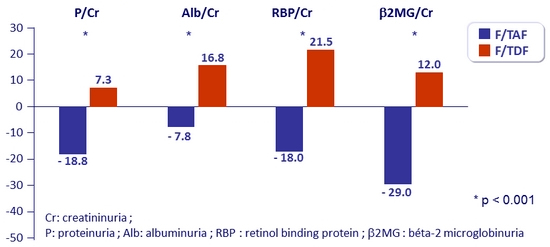
- Median change in eGFR : + 4.5 mg/ dL on RPV/FTC/TAF vs + 0.7 mg/ dL on RPV/FTC/TDF (p = 0.0024)
- No discontinuation for study-drug renal adverse event in either group
- No reported cases of proximal renal tubulopathy or Fanconi syndrome in either group
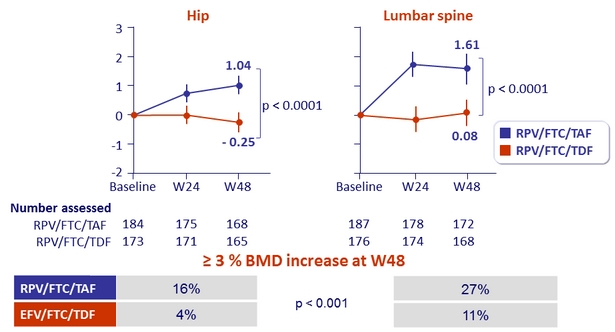
Mean % change in bone mineral density through W48 (%, 95% CI)
Fasting lipids changes at W48
- Increases in total cholesterol, direct LDL, HDL and triglycerides in the RPV/FTC/TAF group
- Stable in the RPV/FTC/TDF group
- Change in total cholesterol:HDL-cholesterol ratio: similar in both groups
- Introduction of lipid-lowering agent between baseline and W48: 4% in the RPV/FTC/TAF group vs 1% in the RPV/FTC/TDF group (p = 0.067)