Pozniak A. JAIDS 2016;71:530-7
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to E/C/F/TAF
Switch studies in virologically suppressed patients
» Switch to E/C/F/TAF
Drugs
E/C/F/TAF, FTC/TAF
E/C/F/TAF, FTC/TAF
- In virologically suppressed HIV-infected subjects with renal impairment, switch to E/C/F/TAF was associated with minimal change in eGFR
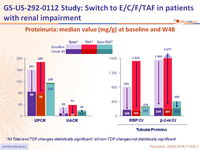
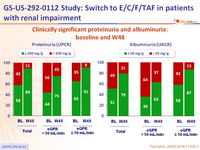
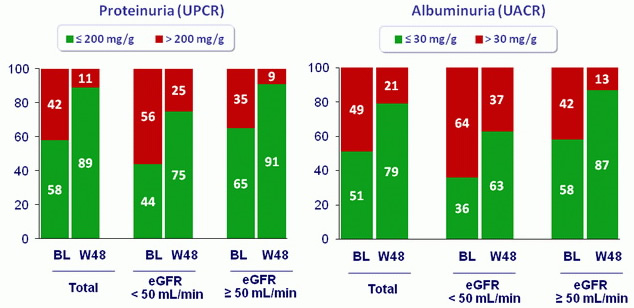
- Proteinuria and albuminuria significantly improved
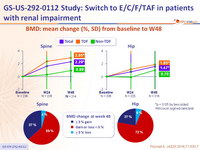
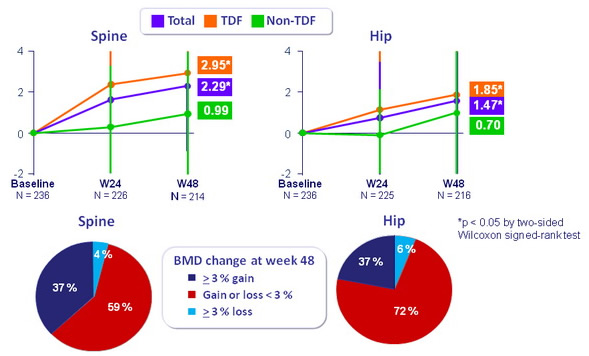
- Bone mineral density significantly improved in patients receiving TDF-containing regimens prior to switching to E/C/F/TA
- Virologic success was maintained in 92% of patients
- These data support the efficacy and safety of once daily E/C/F/TAF in HIV-1 infected patients with mild (eGFR 50-69 mL/min) or moderate (eGFR 30-49 mL/min) renal impairment without dose adjustment
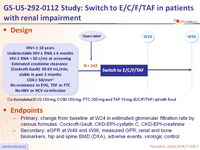
Design
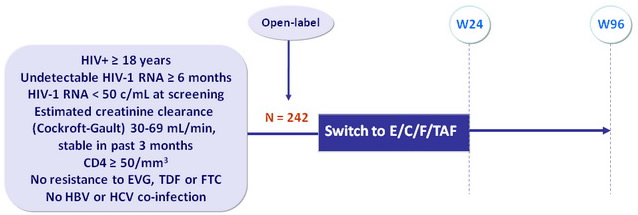
Co-formulated EVG 150 mg, COBI 150 mg, FTC 200 mg and TAF 10 mg (E/C/F/TAF) qd with food
Endpoints
- Primary: change from baseline at W24 in estimated glomerular filtration rate by various formulas: Cockroft-Gault, CKD-EPI-cystatin C, CKD-EPI-creatinine
- Secondary: eGFR at W48 and W96, measured GFR, renal and bone biomarkers, hip and spine BMD (DXA), adverse events, virologic control
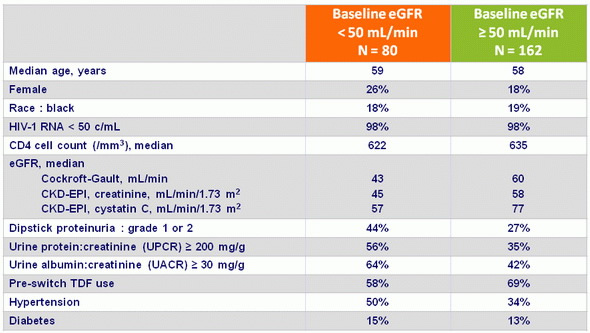
Baseline characteristics
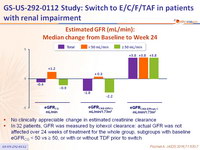
Estimated GFR (mL/min): Median change from Baseline to Week 24
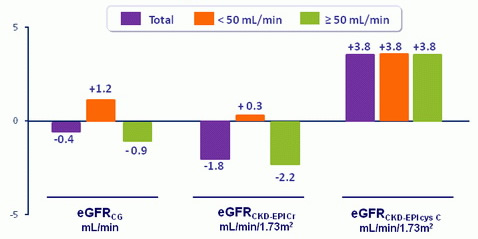
- No clinically appreciable change in estimated creatinine clearance
- In 32 patients, GFR was measured by iohexol clearance: actual GFR was not affected over 24 weeks of treatment for the whole group, subgroups with baseline eGFRCG < 50 vs ≥ 50, or with or without TDF prior to switch
Proteinuria: median value (mg/g) at baseline and W48
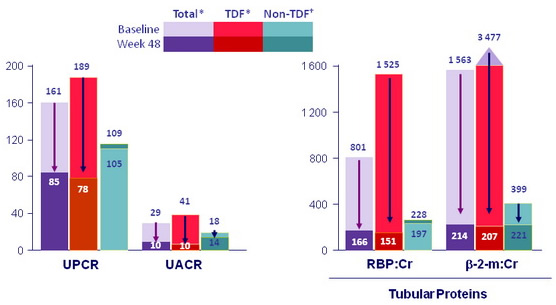
*All Total and TDF changes statistically significant; †all non-TDF changes not statistically significant.
Clinically significant proteinuria and albuminuria: baseline and W48
BMD: mean change (%, SD) from baseline to W48
Adverse events
- Most common adverse events
- Diarrhea 11%
- Upper respiratory tract infection 9%
- Arthralgia 9%
- Bronchitis 8%
- Osteopenia 8%
- Nausea 8%
- Headache 7%
- Pain in extremity 7%
- Back pain 7%
- Dizziness 6%
- Fatigue 6%
- Renal cyst 6%
- Cough 6%
- Adverse events leading to study drug discontinuation, N = 8 (3%), 2/8 for decreased GFR (both patients with hypertension)
- Fractures, N = 6, all related to mechanical trauma
Fasting lipid changes
- Decrease in patients who used non-TDF-containing regimens prior to switching to E/C/F/TAF
- Increase in those using TDF-containing regimens prior to E/C/F/TAF
Pharmacokinetics
- EVG and COBI: in the range of historical data in patients without renal impairment
- FTC: higher than historical data in patients without renal impairment, higher in patients with eGFR < 50 mL/min vs ≥ 50 mL/min
- TAF: consistent with historical data in patients without renal impairment
- TFV: higher than historical data in patients without renal impairment, but well below the TFV exposures from TDF-containing regimens
Efficacy at W48
- HIV-1 RNA < 50 c/mL: 92%
- Virologic failure: 1% (N = 3)