Mills A. Lancet Infect Dis. 2016 Jan;16(1):43-52 & IAS 2015, Abs. TUAB0102 ; DeJesus E. ASM Microbe 2016, Abs. LB-087
Switch studies in virologically suppressed patients
» Switch TDF to TAF
» E/C/F/TAF
E/C/F/TAF, E/C/F/TDF, EVG/c, ATV/r, EFV, FTC/TAF, FTC/TDF, TAF, TDF, FTC
- Switching virologically suppressed patients to TAF regimen
(EVG 150 mg, cobicistat 150 mg, emtricitabine 200 mg, and
TAF 10 mg) was non inferior to the to 3 TDF-containing regimens at week 48 and week 96 - It was also superior to continuing with the TDF-containing regimens.
- Switching to the TAF group showed several important safety advantages of TAF over any of the TDF-containing regimens :
- Improvements in hip and spine bone mineral density
- Improvements in renal function
- Decreases in serum creatinine in patients switched from a boosted regimen
- Decreases in dipstick proteinuria, in quantitative tests of total urine protein, and total urine albumin, in specific proximal tubular proteins
- Improvements in proximal renal tubular function (fractional excretion of uric acid, fractional excretion of phosphate, and renal tubular maximum reabsorption rate of phosphate to the glomerular filtration rate
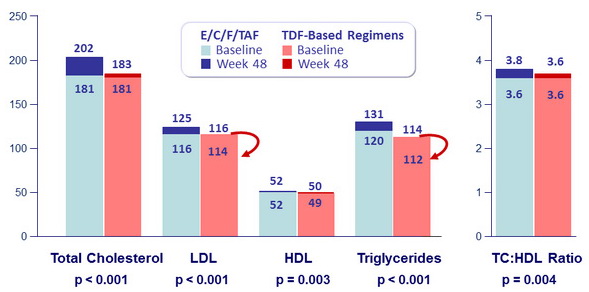
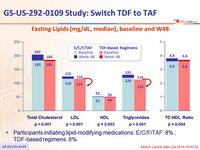
- Slightly higher li pid concentrations with TAF than with TDF-based regimens
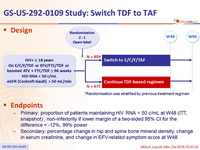
Design

*Randomisation was stratified by previous treatment regimen
Endpoints
- Primary: proportion of patients maintaining HIV RNA < 50 c/mL at W48 (ITT, snapshot) ; non-inferiority if lower margin of a two-sided 95% CI for the difference = -12%, 99% power
- Secondary: percentage change in hip and spine bone mineral density, change in serum creatinine, and change in EFV-related symptom score at W48
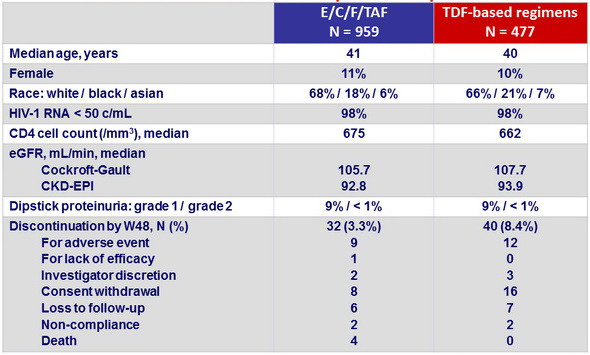
Baseline characteristics and patient disposition
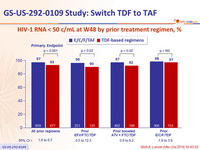
Virologic outcome at W48 (ITT, snapshot)
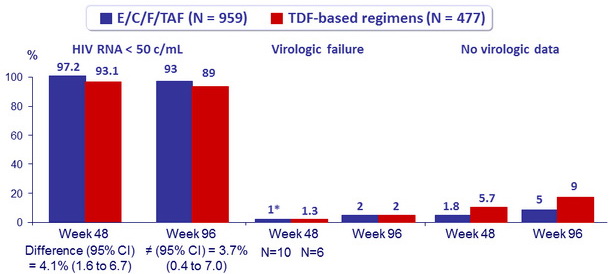
Superiority of E/C/F/TAF
* One patient in the TAF group with virologic failure (W8) had genotypic resistance : M184I/M. The patient resuppressed 4 weeks later without a change of regimen
HIV-1 RNA < 50 c/mL at W48 by prior treatment regimen, %
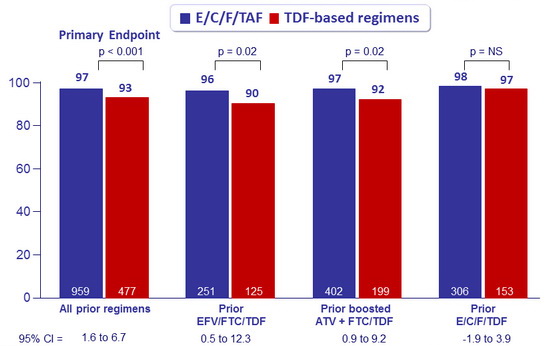
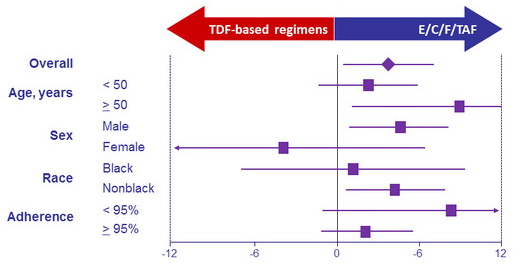
Differences in patients with HIV-1 RNA < 50 c/mL at W96 by subgroup, % (95% CI)
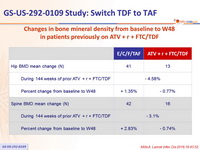
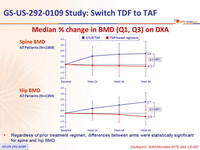
Changes in bone mineral density from baseline to W48
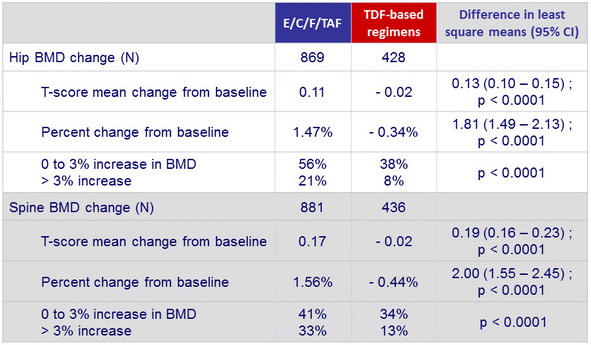
Changes in bone mineral density from baseline to W48
in patients previously
on ATV + r + FTC/TDF
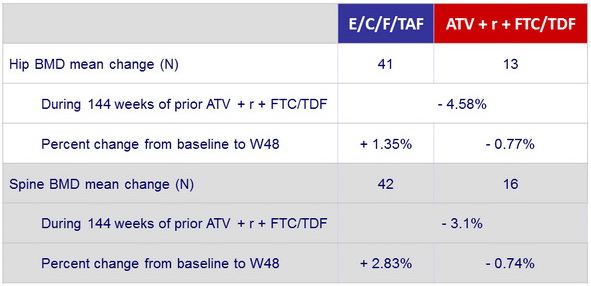
Median % change in BMD (Q1, Q3) on DXA
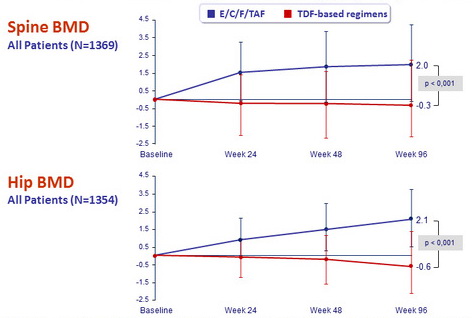
- Regardless of prior treatment regimen, differences between arms were statistically significant for spine and hip BMD
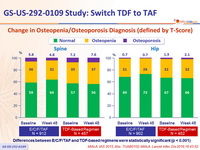
Change in Osteopenia/Osteoporosis Diagnosis (defined by T-Score)
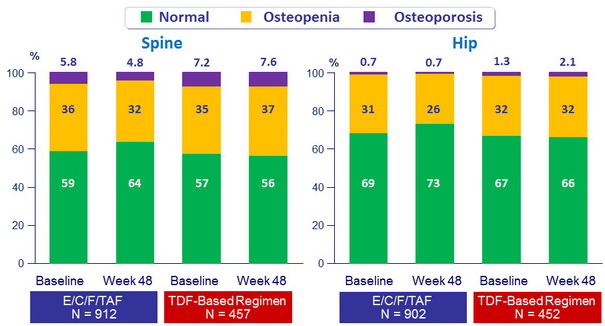
Differences between E/C/F/TAF and TDF-based regimens were statistically significant (p < 0.001)
Serum creatinine and eGFR changes from baseline to W48
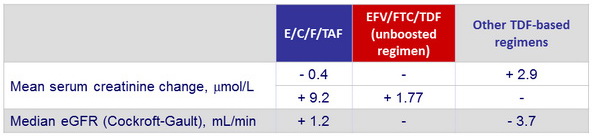
Median changes in markers of renal function from baseline to W48

* TmP /GFR: ratio of tubular maximum reabsorption of phosphate ( TmP ) to GFR
Renal Safety Results (Median % change in proteinuria at W48)
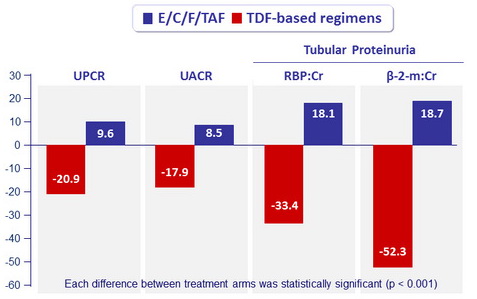
UPCR: urine protein:creatinine ratio ; UACR: urine albumin:creatinine ratio ;
RBP, retinol-binding protein ; β-2- m: beta-2 microglobulin
Adverse events, N (%) (W48)
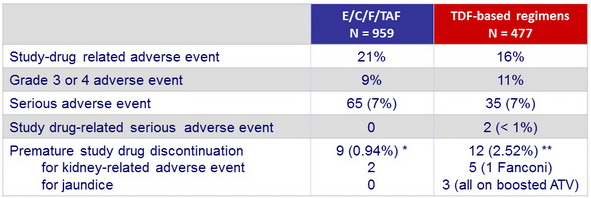
* Panic attack; apathy, amnesia, speech disorder; Reiter's syndrome; nausea, vomiting, and headache; suicide attempt; leg swelling and impaired concentration; depression; acute renal failure (a fter cancer chemotherapy: sepsis and multisystem organ failure) ; tubulo -interstitial nephritis (recurrent hematuria with subsequent diagnosis of Hodgkin lymphoma)
** Abnormal dreams; depression, insomnia, and irritability; depression, insomnia, and nightmares; elevated bilirubin; jaundice (N = 2); memory impairment; chronic kidney disease; elevated serum creatinine (N = 2); Fanconi's syndrome and mild jaundice; renal colic
- No discontinuation for adverse event in both groups between W48 and W96
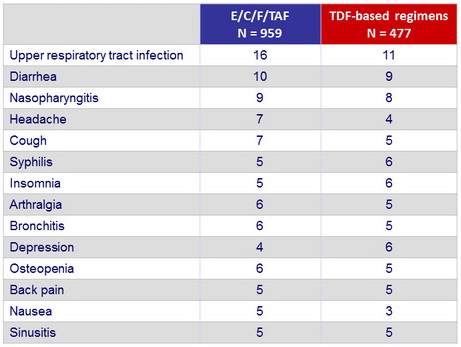
Most-common drug-related adverse event, % (W48)
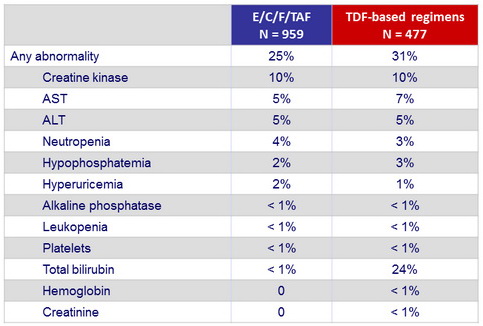
Grade 2-4 laboratory abnormalities (W48)
Fasting Lipids (mg/dL, median), baseline and W48