Gallant J. CROI 2016, Abs. 29 & Lancet HIV. 2016;3:e158-65 ; Raffi F. J Acquir Immune Defic Syndr. 2017;75:226-31
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch TDF to TAF
» FTC/TDF vs FTC/TAF
Switch studies in virologically suppressed patients
» Switch TDF to TAF
» FTC/TDF vs FTC/TAF
Drugs
FTC/TAF, FTC/TDF, TAF, TDF, FTC
FTC/TAF, FTC/TDF, TAF, TDF, FTC
- In this randomised double-blind study, switch of patients on F/TDF
+ 3rd agent with suppressed viral load for F/TAF + continuation
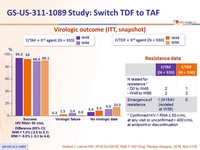
of 3rd agent- Is non-inferior at W48 and W96 for maintaining virologic suppression (HIV-1 RNA < 50 c/mL)
- in rare cases of virologic failure, the risk of resistance emergence is low (1 case of M184V on F/TAF), none after W48
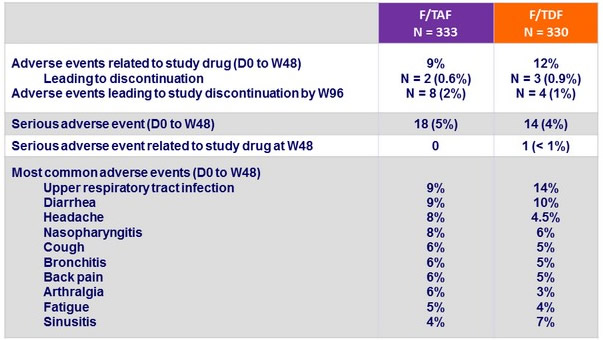
- Is associated with a similar clinical and biological tolerance
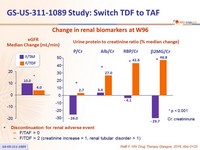
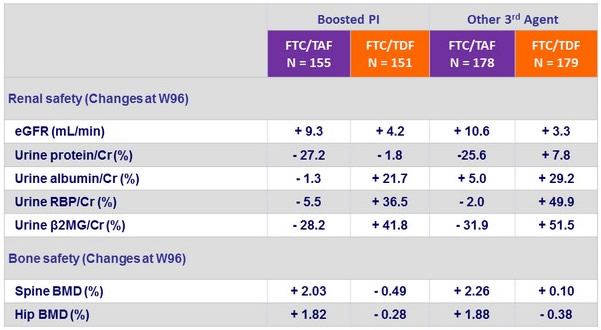
- Leads to improvement of renal parameters: increase in eGFR and decrease in proteinuria, n o renal discontinuations or renal tubulopathy
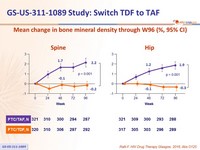
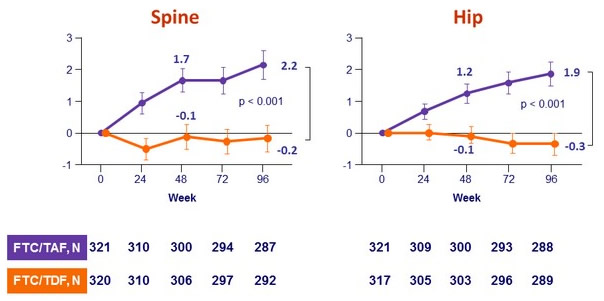
in F/TAF group - Improves bone mineral density: increase on F/TAF with significant difference of changes at W48 vs F/TDF and c ontinuing increase in hip and spine BMD after W48
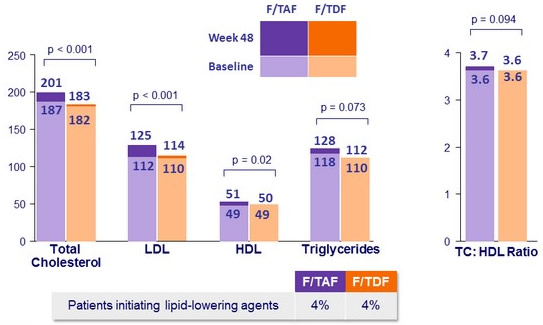
- Increases lipid parameters, with no change in the total cholesterol total: HDL-cholesterol ratio
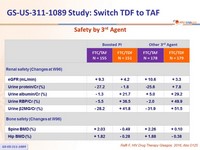
- Similar safety findings by 3rd agent
Design
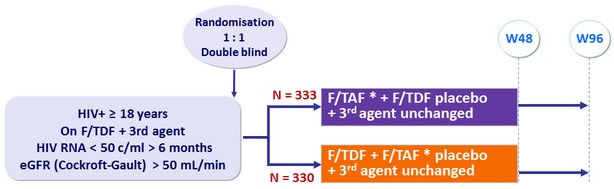
Randomisation stratified on 3rd agent (boosted PI or other)
* F/TAF: 200/10 mg if boosted PI, 200/25 mg if other
Endpoints
- Primary: proportion of patients maintaining HIV RNA < 50 c/mL at W48 (ITT, snapshot) ; non-inferiority if lower margin of a two-sided 95% CI for the difference = -10%, > 95% power
- Secondary with multiple adjustments: percentage change in hip and spine bone mineral density
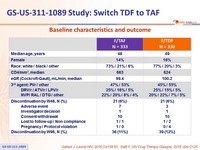
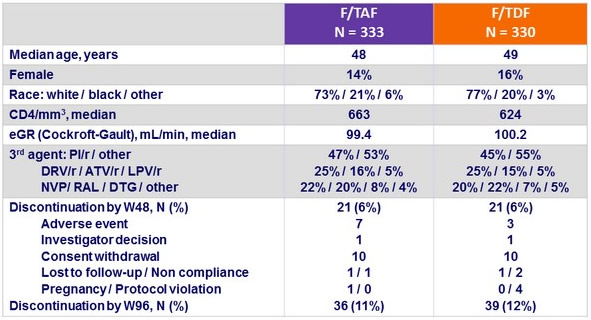
Baseline characteristics and outcome
Virologic outcome at W48 (ITT, snapshot)
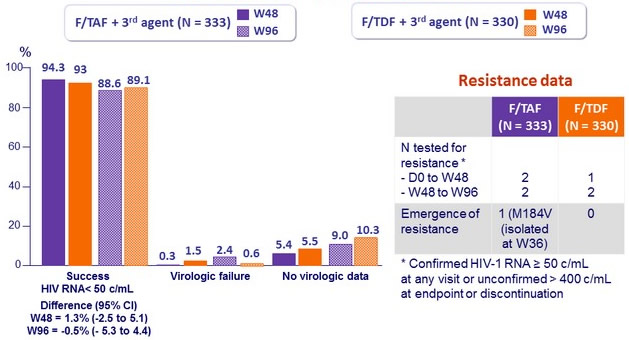
Difference (95% CI)
= 1.3% (-2.5 to 5.1)
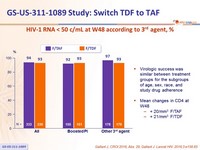
HIV-1 RNA < 50 c/mL at W48 according to 3rd agent, %
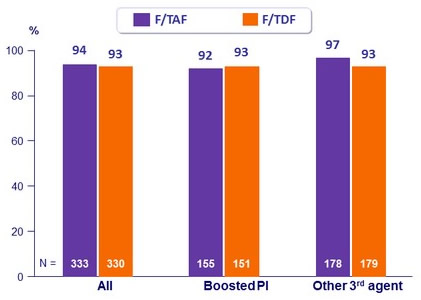
- Virologic success was similar between treatment groups for the subgroups of age, sex, race, and study drug adherence
- Mean changes in CD4 at W48 ‒
- + 20/mm3 F/TAF
- + 21/mm3 F/TDF
Adverse events, N (%)
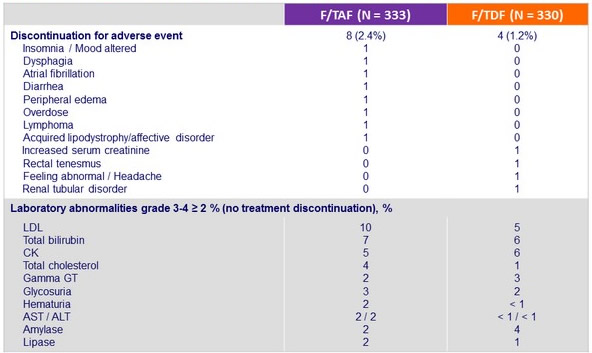
Discontinuation for adverse event, laboratory abnormalities grade 3-4 (D0-W96)
Mean change in bone mineral density through W96 (%, 95% CI)
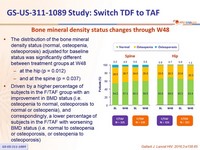
Bone mineral density status changes through W48
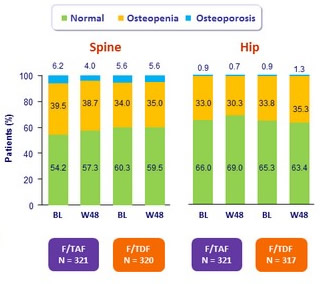 The distribution of the bone mineral density status (normal, osteopenia, osteoporosis) adjusted for baseline status was significantly different between treatment groups at W48
The distribution of the bone mineral density status (normal, osteopenia, osteoporosis) adjusted for baseline status was significantly different between treatment groups at W48
- at the hip (p = 0.012)
- and at the spine (p = 0.037)
- Driven by a higher percentage of subjects in the F/TAF group with an improvement in BMD status (i.e. osteopenia to normal, osteoporosis to normal or osteopenia), and correspondingly, a lower percentage of subjects in the F/TAF with worsening BMD status (i.e. normal to osteopenia or osteoporosis, or osteopenia to osteoporosis)
Change in renal biomarkers at W96
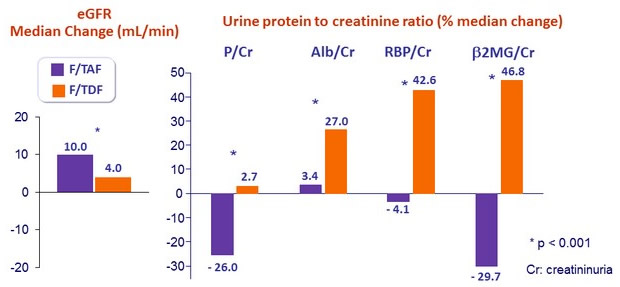
- Discontinuation for renal adverse event:
- F/TAF = 0
- F/TDF = 2 (creatinine increase = 1, renal tubular disorder = 1)
Safety by 3rd Agent
Median fasting lipids W48 versus baseline (mg/dL)