Winston A. Lancet HIV.2018 ; 5:e162-71
Switch studies in virologically suppressed patients
» Switch ABC/3TC to TDF/FTC
» ABC/3TC vs FTC/TAF
FTC/TAF, ABC/3TC, TAF, ABC, FTC, 3TC
- Switch from ABC/3TC to TAF/FTC was non inferior to ABC/3TC in maintaining virologic suppression in combination with a variety of third agents
- No differences of TAF/FTC vs ABC/3TC in
- Renal biomarkers
- Bone mineral density
- Fasting lipids
- In virologically suppressed patients with creatinine clearance > 50 mL/min, TAF/FTC provides an alternative backbone to ABC/3TC with similar effects on kidney and bone
Design
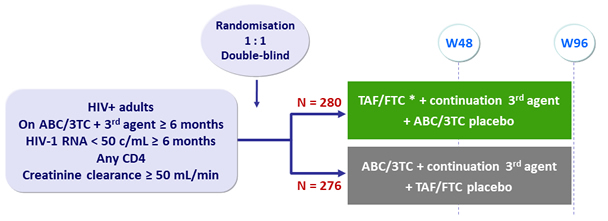
* TAF/FTC : 100/200 mg when coadministered with ATV boosted, DRV boosted or LPV/r ; 25/200 mg when co-administered with NNRTI, RAL, DTG or MVC
Objective
- Primary endpoint: non-inferiority of TAF/FTC at W48: % HIV-1 RNA < 50 c/mL by ITT, snapshot analysis ; lower limit of the 95% CI for the difference = - 10%, 90% power
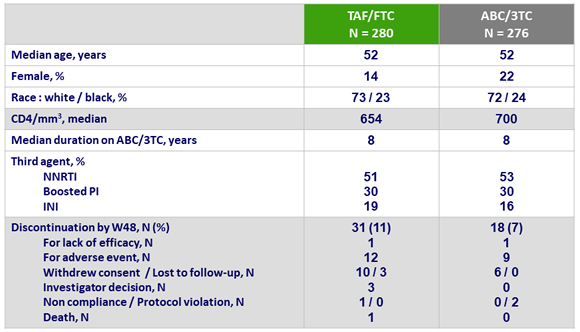
Baseline characteristics and patient disposition
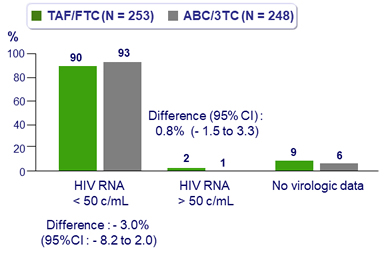
Efficacy at W48 (ITT, snapshot)
Per protocol analysis
- HIV RNA < 50 c/mL at W48
- TAF/FTC : 99.1%
- ABC/3TC : 99.1%
- Difference : 0.0% (95% CI: -2.5 to 2.3)
Emergence of resistance, N
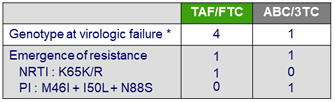
* Confirmed HIV RNA > 50 c/ mL or unconfirmed > 400 c/ mL at last visit
Adverse events
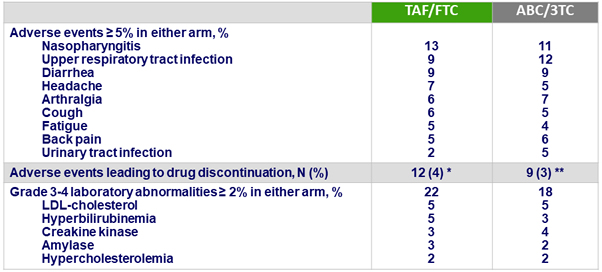
*
Considered related to study drug
(n = 8) : abdominal distension + myalgia, nausea + vomiting + dehydration, nausea + feeling jittery + decrease appetite, anxiety, Brugada syndrome, increased creatinine, burning sensation + headache + paresthesia, vision blurred + visual field defect + eye pain ; not related to study drug : cough, tuberculosis, sudden cardiac death, neutropenia
**
Considered related to study drug
(n = 8) : affective disorder, panic attack, depression + bone pain + arthralgia, dermatitis + pruritus, rash + pruritus, diarrhea, myalgia + dysesthesia, tinnitus + dry mouth + dyspnea ; not related to study drug : depression + suicide attempt
Median changes in creatinine clearance (Q1, Q3), mL/min
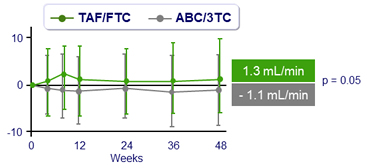
No difference between groups in changes at W48
- -In renal biomarkers (urine RBP:creatinine and B2M:creatinine ratios)
- In bone mineral density (hip and lumbar spine)
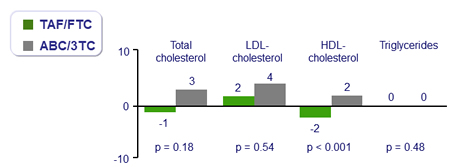
Median changes in fasting lipids at W48, mg/dL