Trottier B. Antivir Ther. 2017;22(4):295-305
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG /ABC/3TC vs 2 NRTI + 3rd agent
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG /ABC/3TC vs 2 NRTI + 3rd agent
Drugs
DTG, 2 NRTI, ABC/3TC, ABC, 3TC
DTG, 2 NRTI, ABC/3TC, ABC, 3TC
- Efficacy
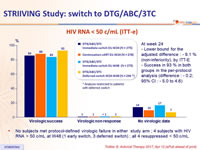
- Similar virologic response for DTG/ABC/3TC and continuation of current ART at W24, with non-inferiority
- Success rate was maintained through 48 weeks in the early switch group
- In the late switch group, virologic suppression was observed in 92% of subjects on DTG/ABC/3TC (24 weeks post-switch)
- There were no protocol-defined virologic failure in the study
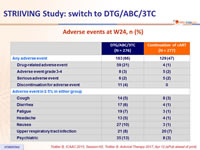
- Tolerability
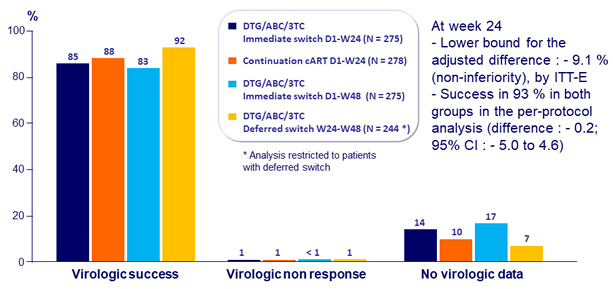
- 4% of subjects discontinued due to adverse events by W24 in the DTG/ABC/3TC arm vs 0% in the continuation group
- There were no further discontinuations due to adverse events in the early switch arm post-week 24
- Rates of discontinuation for adverse events in the late switch arm was 2%
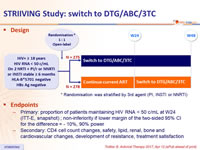
Design
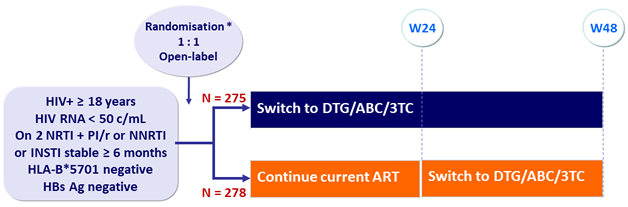
* Randomisation was stratified by 3rd agent (PI, INSTI or NNRTI)
Endpoints
- Primary: proportion of patients maintaining HIV RNA < 50 c/mL at W48 (ITT-E, snapshot) ; non-inferiority if lower margin of the two-sided 95% CI for the difference = - 10%, 90% power
- Secondary: CD4 cell count changes, safety, lipid, renal, bone and cardiovascular changes, development of resistance, treatment satisfaction
Baseline characteristics and patient disposition
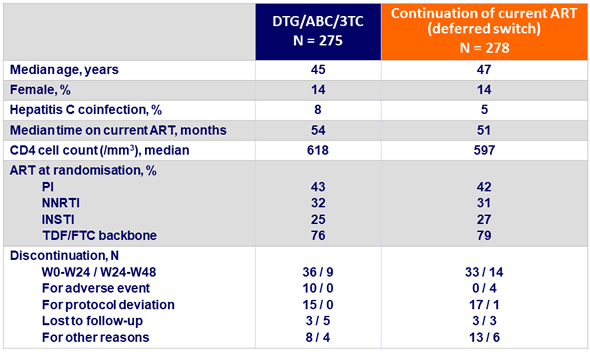
HIV RNA < 50 c/ mL (ITT-e)
- No subjects met protocol-defined virologic failure in either study arm ; 4 subjects with HIV RNA > 50 c/mL at W48 (1 early switch, 3 deferred switch) ; all 4 resuppressed < 50 c/mL
Adverse events at W24, n (%)
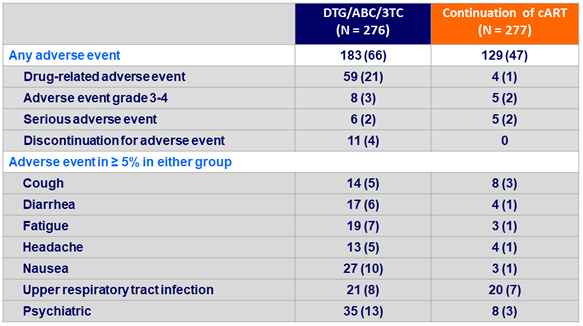
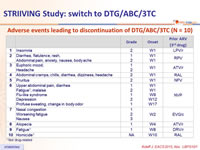
Adverse events leading to discontinuation of DTG/ABC/3TC (N = 10)
1 Not drug related
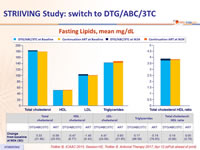
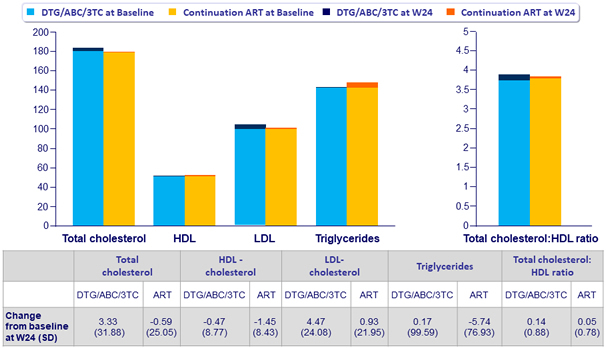
Fasting Lipids, mean mg/ dL
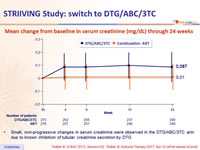
Mean change from baseline in serum creatinine (mg/dL) through 24 weeks
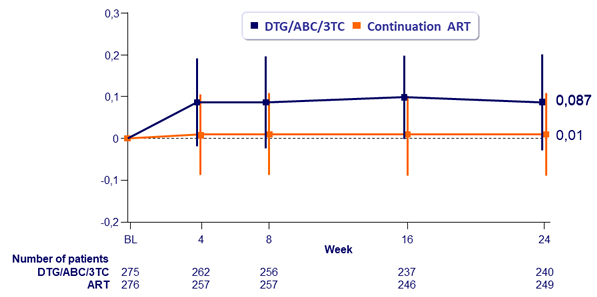
- Small, non-progressive changes in serum creatinine were observed in the DTG/ABC/3TC arm due to known inhibition of tubular creatinine secretion by DTG
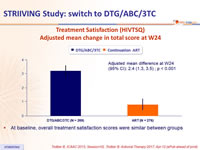
Treatment Satisfaction (HIVTSQ)
Adjusted mean change in total score at W24
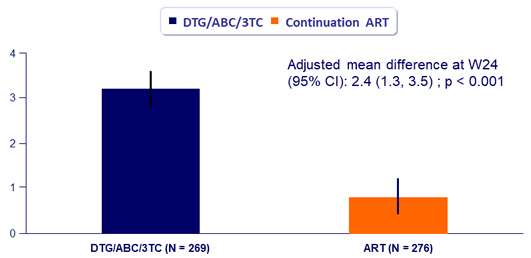
- At baseline, overall treatment satisfaction scores were similar between groups