Arribas JR. Lancet Infect Dis. 2014 Jul;14(7):581-9
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to E/C/FTC/TDF
» EVG/C/F/TDF vs PI/r + FTC/TDF
Switch studies in virologically suppressed patients
» Switch to E/C/FTC/TDF
» EVG/C/F/TDF vs PI/r + FTC/TDF
Drugs
E/C/F/TDF, EVG/c, DRV/r, ATV/r, FPV/r, NVP, EFV, FTC/TDF, TDF, FTC
E/C/F/TDF, EVG/c, DRV/r, ATV/r, FPV/r, NVP, EFV, FTC/TDF, TDF, FTC
- Coformulated EVG/c/FTC/TDF is an effective, safe, and tolerable simplification from a PI/r plus FTC and TDF regimen in virologically suppressed, HIV-infected adults with no history of virological failure or resistance to FTC or TDF
- Low frequency of virologic failure and absence of emergent resistance in the group switched to EVG/c/FTC/TDF
- Rare discontinuations because of adverse events
- Nausea more frequent in the switch group ; diarrhea and bloating improved
- Small increase in creatinine, moderate improvement in lipids
- EVG/c/FTC/TDF is a switch option in virologically suppressed patients with no history of virological failure who want to simplify their existing PI/r regimen, or who have concerns about the long-term safety and side-effects of their existing regimen
Design :
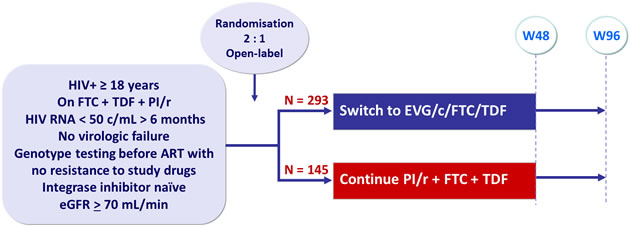
Endpoints :
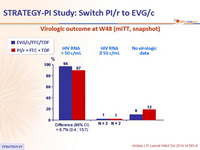
- Primary: proportion of patients maintaining HIV RNA < 50 c/mL at W48 (mITT, snapshot) ; non-inferiority if lower margin of a two-sided 95% CI for the difference = -12%, 85% power. If non-inferiority and lower margin > 0, assessment for superiority
- Secondary: proportion of patients maintaining HIV RNA < 50 c/mL at W48 (TLOVR algorithm), CD4, safety, tolerability to W96
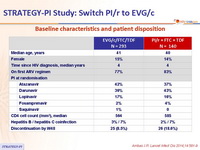
Baseline characteristics and patient disposition :
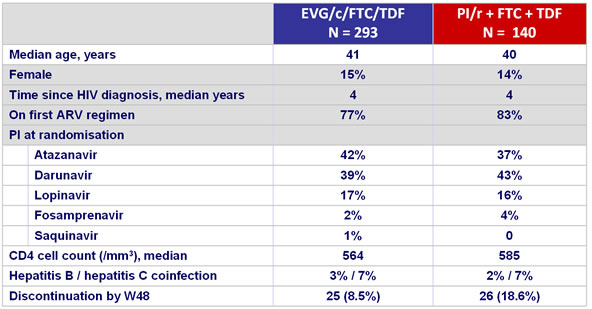
Virologic outcome at W48 (mITT, snapshot)
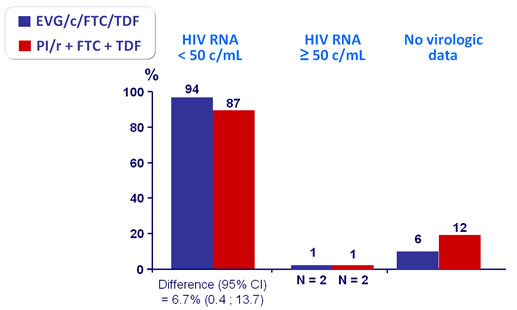
HIV RNA < 50 c/mL - Sensitivity and secondary analysis :
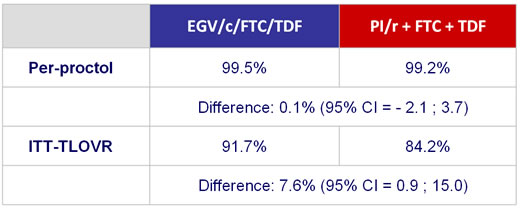
- No participants met the criteria for resistance testing (HIV RNA > 400 c/mL at virologic failure or early discontinuation)
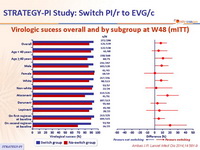
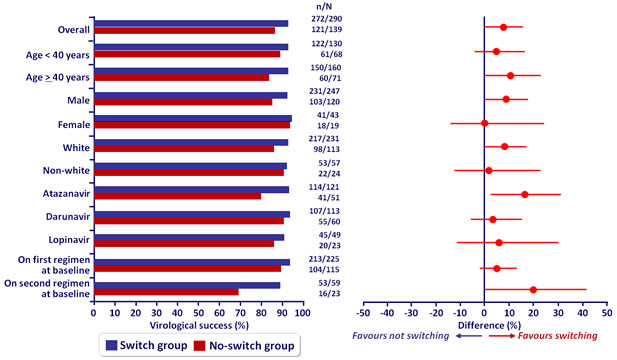
Virologic sucess overall and by subgroup at W48 (mITT) :
Adverse events and grade3-4 laboratory abnormalities :
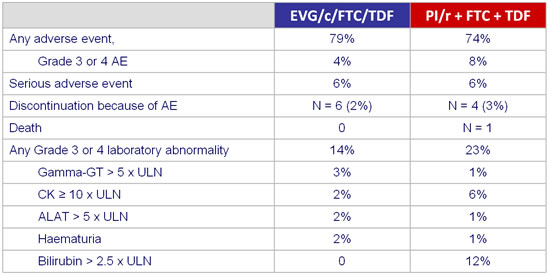
- Improvement in lipids in the switch group
- HIV Symptom Index : rates of diarrhea and bloating decreased in the switch group
- Higher tretament satisfaction scores in the switch group