Pozniak A. Lancet Infect Dis. 2014 Jul;14(7):590-9
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to E/C/FTC/TDF
» EVG/C/F/TDF vs NNRTI + FTC/TDF
Switch studies in virologically suppressed patients
» Switch to E/C/FTC/TDF
» EVG/C/F/TDF vs NNRTI + FTC/TDF
Drugs
E/C/F/TDF, EVG/c, FTC/TDF, TDF, FTC
E/C/F/TDF, EVG/c, FTC/TDF, TDF, FTC
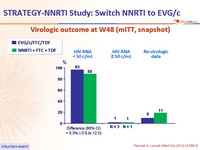
- Coformulated EVG/c/FTC/TDF is non-inferior to continuing an existing regimen of NNRTI plus FTC and TDF in virologically suppressed, HIV-infected adults with no history of virological failure or resistance to FTC or TDF.
- Low frequency of virologic failure and absence of emergent resistance in the group switched to EVG/c/FTC/TDF
- Rare discontinuations because of adverse events
- Fatigue, cough, headache and nausea were more frequent in the switch group ;
- Rates of CNS symptoms decreased in patients switched from EFV
- Increase in creatinine similar to that of phase 3 of EGV/c/FTC/TDF
- Moderate improvement in lipids in patients switched from EFV
- EVG/c/FTC/TDF is a switch option in virologically suppressed patients with no history of virological failure on an NNRTI regimen, when its continuation is not suitable
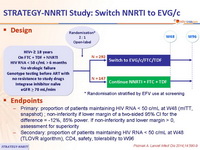
Design :
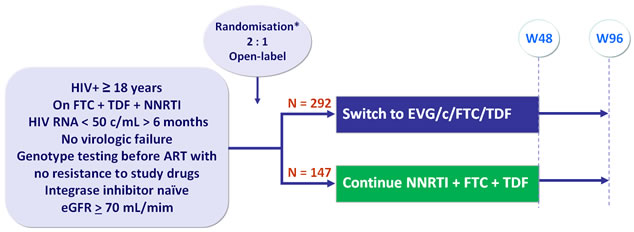
Endpoints :
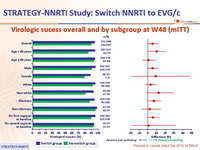
- Primary: proportion of patients maintaining HIV RNA < 50 c/mL at W48 (mITT, snapshot) ; non-inferiority if lower margin of a two-sided 95% CI for the difference = -12%, 85% power. If non-inferiority and lower margin > 0, assessment for superiority
- Secondary: proportion of patients maintaining HIV RNA < 50 c/mL at W48 (TLOVR algorithm), CD4, safety, tolerability to W96
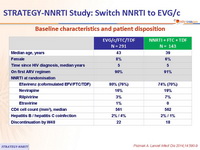
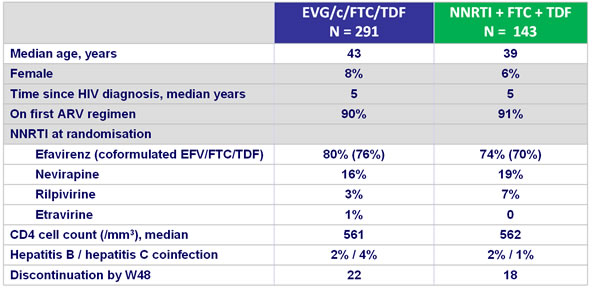
Baseline characteristics and patient disposition :
Virologic outcome at W48 (mITT, snapshot)
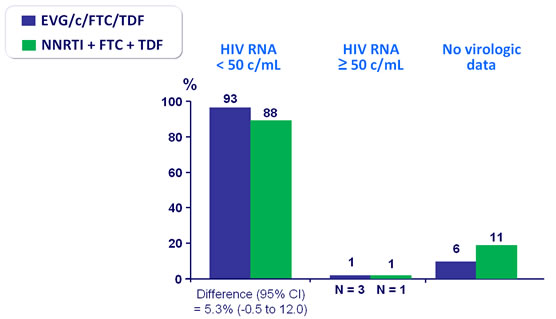
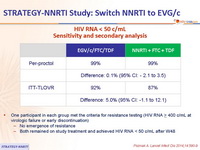
HIV RNA < 50 c/mL - Sensitivity and secondary analysis :
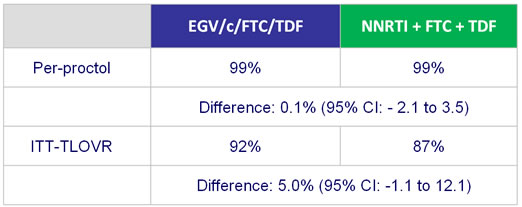
- One participant in each group met the criteria for resistance testing (HIV RNA > 400 c/mL at virologic failure or early discontinuation)
- No emergence of resistance
- Both remained on study treatment and achieved HIV RNA < 50 c/mL after W48
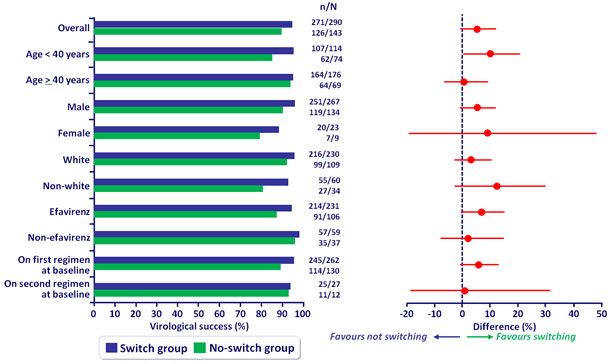
Virologic sucess overall and by subgroup at W48 (mITT) :
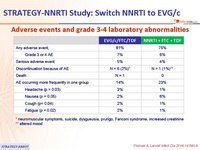
Adverse events and grade3-4 laboratory abnormalities :
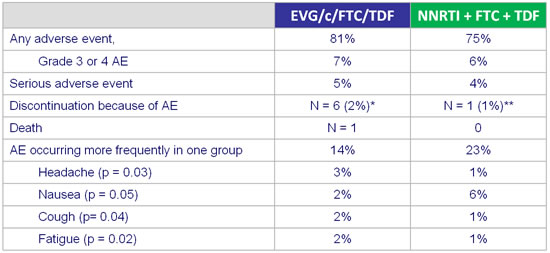
* neuromuscular symptoms, suicide, dysgueusia, prurigo, Fanconi syndrome, increased creatinine
** altered mood
Other safety data :
- Incidence and prevalence of headache and nausea became similar between groups by week 12
- Grade 3-4 laboratory abnormalities : 10% in the switch group vs 14% in the no-switch group
- Creatinine increase in switch group at week 4, stabilizing up to week 48 (median +11 mmol/L)
- Small decrease in HDL-cholesterol in the switch group vs no change in the no-switch group
- In the subgroup switched form EFV + FTC + TDF to EVG/c/FTC/TDF
- Improvement in lipids
- HIV Symptom Index : improvement of CNS symptoms
- Higher treatment satisfaction score at W4 and W24