Raffi F. Lancet. 2013 Mar 2;381(9868):735-43; Lancet Infect Dis. 2013 Nov;13(11):927-35
Head-to-head comparative trials for first line ART since 2006
» INSTI vs INSTI
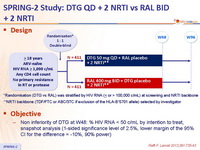
» DTG QD + 2 NRTI vs RAL BID + 2 NRTI
DTG, RAL, 2 NRTI
- DTG 50 mg QD was virologically non-inferior to RAL BID, (both + 2 NRTIs) over 48 and 96 weeks
- No INSTI mutations were detected through 96 weeks with DTG
- DTG was similar to RAL in terms of safety and tolerability
- Low occurrence of adverse events leading to discontinuation : 2% in each group
- Between W48 and W96 : few new virologic failures and few discontinuations for adverse events
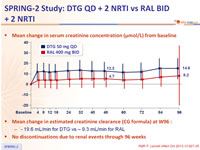
- No discontinuation due to renal events through 96 weeks
- Mean increases in creatinine with accompanying decreases in estimated glomerular filtration rate
- occurred in both study groups by week 4
- generally stabilised and did not change up to week 96
Design :
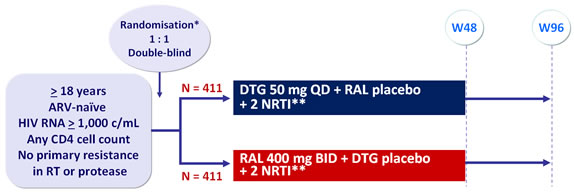
*Randomisation (DTG vs RAL) was stratified by HIV RNA (≤ or > 100,000 c/mL) at screening and NRTI backbone
**NRTI backbone (TDF/FTC or ABC/3TC if exclusion of the HLA-B*5701 allele) selected by investigator
Objective :
- Non inferiority of DTG at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (1-sided significance level of 2.5%, lower margin of the 95% CI for the difference = -10%, 90% power)
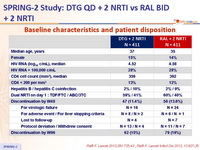
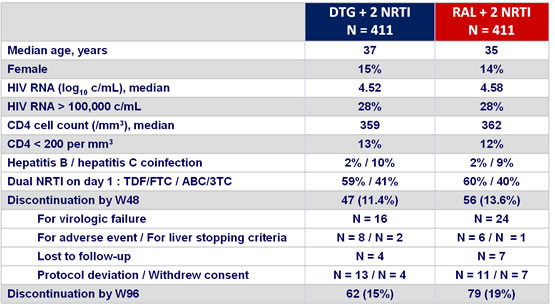
Baseline characteristics and patient disposition :
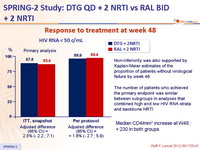
Response to treatment at week 48 :
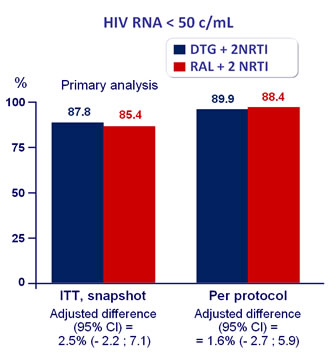
Non-inferiority was also supported by Kaplan-Meier estimates of the proportion of patients without virological failure by week 48
The number of patients who achieved the primary endpoint was similar between subgroups in analyses that combined high and low HIV RNA strata and backbone NRTI
Median CD4/mm3 increase at W48 :
+ 230 in both groups
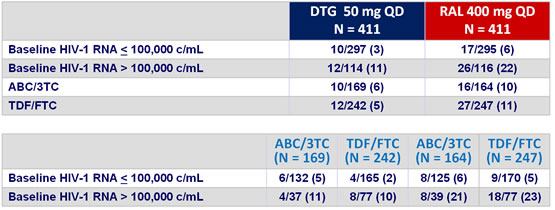
HIV-1 RNA < 50 c/mL at week 48 by stratification factors :
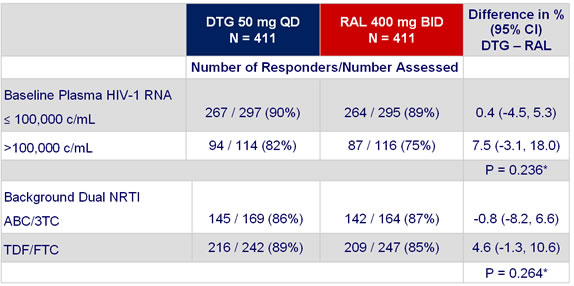
* Test for homogeneity
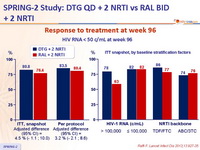
Response to treatment at week 96 :
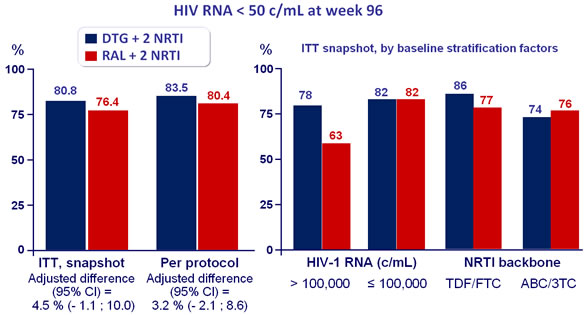
Virologic non-responders (ITT, snapshot) at week 96 by baseline
stratification factors :
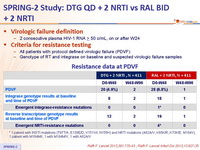
Virologic failure definition :
- 2 consecutive plasma HIV-1 RNA > 50 c/mL, on or after W24
Criteria for resistance testing
- All patients with protocol defined virologic failure (PDVF)
- Genotype of RT and integrase on baseline and suspected virologic failure samples
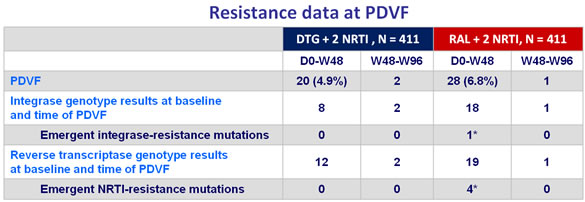
* 1 patient with INSTI mutations (T97T/A, E138E/D, V151V/I, N155H) and NRTI mutations (A62A/V, K65K/R, K70K/E, M184V), 1 patient with M184M/I, 1 with M184M/V, 1 with A62A/V
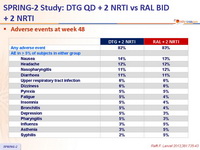
Adverse events at week 48 :
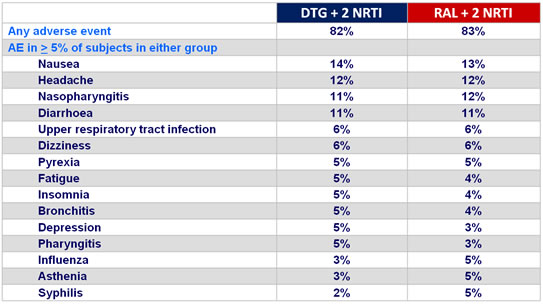
Graded laboratory toxic effects : rates similar between groups
Serious adverse events at week 48
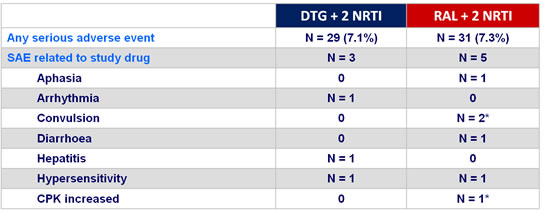
* 1 patient with elevated CPK + convulsion
Safety between W48 and W96 :
- Adverse events leading to discontinuation : 0 for DTG vs 3 for RAL
- No serious adverse events related to study drugs
Mean change in serum creatinine concentration (µmol/L) from baseline :
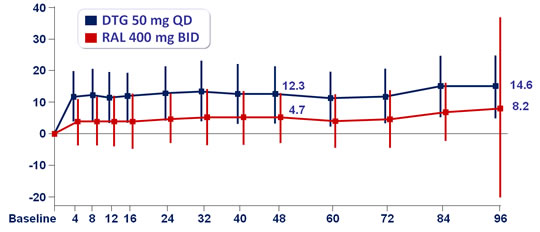
Mean change in estimated creatinine clearance (CG formula) at W96 :
- - 19.6 mL/min for DTG vs – 9.3 mL/min for RAL