Paella FJ. Jr AIDS. 2014 Jan 28;28(3):335-44
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch PI to NNRTI
» RPV/FTC/TDF
Switch studies in virologically suppressed patients
» Switch PI to NNRTI
» RPV/FTC/TDF
Drugs
PI/r mono, RPV, 2 NRTI, FTC/TDF, TDF, FTC
PI/r mono, RPV, 2 NRTI, FTC/TDF, TDF, FTC
- Switching to the STR TDF/FTC/RPV from a PI/r regimen in virologically suppressed, HIV-1-infected participants maintained virologic suppression with a low risk of virologic failure, while improving total cholesterol, LDL-cholesterol, and triglycerides
- Participants had been virologically suppressed on a PI/r regimen for at least 6 months prior to study entry and had no previous ART failure
- Pretreatment HIV-1 RNA levels (while still ARV-naive) did not affect maintenance of viral suppression after switch to TDF/FTC/RPV
- Historical K103 resistance mutation (probably transmitted) did not affect efficacy of switch to TDF/FTC/RPV in participants of the study
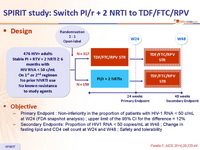
Design :

Objective :
- Primary Endpoint : Non-inferiority in the proportion of patients with HIV-1 RNA < 50 c/mL at W24 (FDA snapshot analysis) ; upper limit of the 95% CI for the difference = 12%
- Secondary Endpoints: Proportion of HIV1 RNA < 50 copies/mL at W48 ; Change in fasting lipid and CD4 cell count at W24 and W48 ; Safety and tolerability
Baseline characteristics and disposition :

ART at screening :

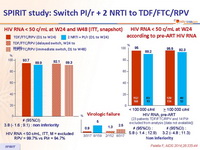
HIV RNA < 50 c/mL at W24 and W48 (ITT, snapshot) :

HIV RNA < 50 c/mL, ITT, M = excluded RPV = 99.7% vs PI/r = 94.7%
HIV RNA < 50 c/mL at W24 according to pre-ART HIV RNA :

Virologic failure

Among the 24 patients with the K103N mutation on historical genotype :
- 18 in the immediate switch arm
- All maintain HIV RNA < 50 c/mL at W24
- 1 virologic failure at W48 (pre-existing mutations : K103N + V179I, emergence : M184V, E138K and V108V/I)
- 6 in the delayed switch arm
- 5 maintain HIV RNA < 50 c/mL at W48 (24 weeks after switch)
- 1 without data at W48 (HIV RNA < 50 c/mL at last study visit)
Virologic failure on TDF/FTC/RPV, N = 7 (1.5%) :
- 3 without emergence of resistance mutations
- 4 with emergence of resistance mutations
- K103N + L100I + M184I
- M184I
- E138E/K + M184M/V
- E138K + V108V/I + M184V
Discontinuation for adverse event (W24) :
- TDF/FTC/RPV, N = 6
- tubulopathy, N = 1
- neuro-psychiatric events, N = 4 (depression, headache, insomnia, psychiatric event)
- 2 NRTI + PI/r, N = 0
GFR decrease significantly more important with RPV
Grade 3-4 Adverse events and laboratoratory abnormalities to W48 :

Mean change from baseline at W24 :