Nishijima T. PLoS One. 2013 Aug 8;8(8)
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to RAL + PI/r
» RAL + DRV/r vs LPV/r + FTC/TDF
Switch studies in virologically suppressed patients
» Switch to RAL + PI/r
» RAL + DRV/r vs LPV/r + FTC/TDF
Drugs
RAL, DRV/r, LPV/r, FTC/TDF, TDF, FTC
RAL, DRV/r, LPV/r, FTC/TDF, TDF, FTC
- Switching LPV/r + TDF/FTC to RAL+ DRV/r did not significantly increase the proportion of patients who showed >10% improvement in renal function among those with relatively preserved eGFR . However, the switch improved urinary β2 microglobulin, suggesting that discontinuation of TDF might be beneficial in the long-term
- RAL +DRV/r showed favorable viral efficacy in patients with suppressed viral load
- Limitations
- Small sample size
- Adverse events self-reported, open-label unblinded design
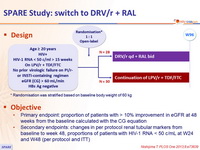
Design
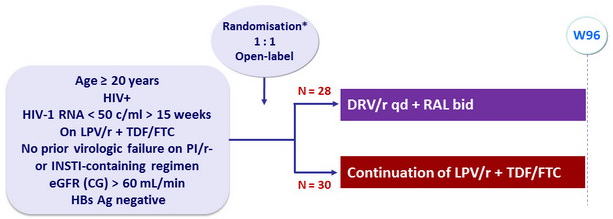
* Randomisation was stratified based on baseline body weight of 60 kg
Objective
- Primary endpoint: proportion of patients with > 10% improvement in eGFR at 48 weeks from the baseline calculated with the CG equation
- Secondary endpoints: changes in per protocol renal tubular markers from baseline to week 48, proportions of patients with HIV-1 RNA < 50 c/mL at W24 and W48 (per protocol and ITT)
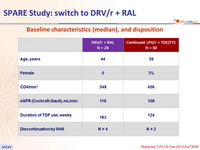
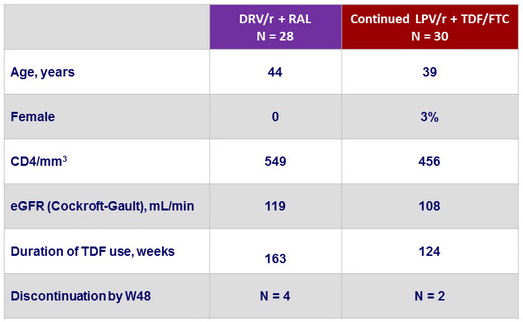
Baseline characteristics (mean), and disposition
Endpoints by W48