Kityo C .PLoS Med. 2018 ; 15(12):e1002706
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» Intensification with INSTI
» EFV + 2 NRTI vs EFV + 2 NRTI + RAL
Head-to-head comparative trials for first line ART since 2006
» Intensification with INSTI
» EFV + 2 NRTI vs EFV + 2 NRTI + RAL
Drugs
RAL, EFV 600, 2 NRTI
RAL, EFV 600, 2 NRTI
- Standard triple ART (FTC/TDF + EFV) intensified with raltegravir
for 12 weeks- Was well tolerated
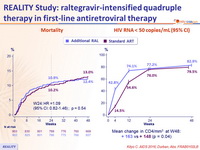
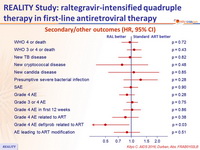
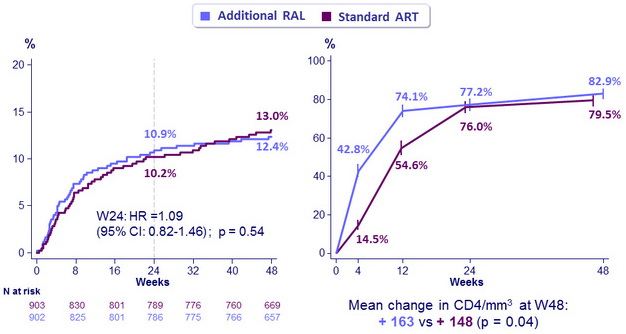
- Resulted in faster HIV RNA reduction through 24 weeks, and higher increase in CD4 at 48 weeks
- But did not reduce mortality or WHO 3/4 events through either week 24 or 48
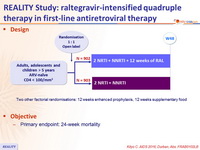
Design
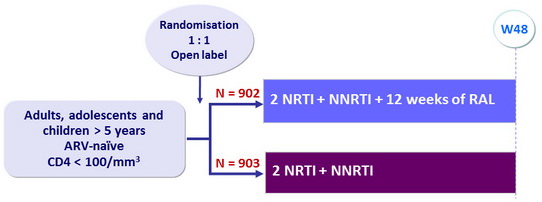
Two other factorial randomisations: 12 weeks enhanced prophylaxis, 12 weeks supplementary food
Objective
- Primary endpoint: 24-week mortality
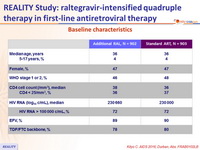
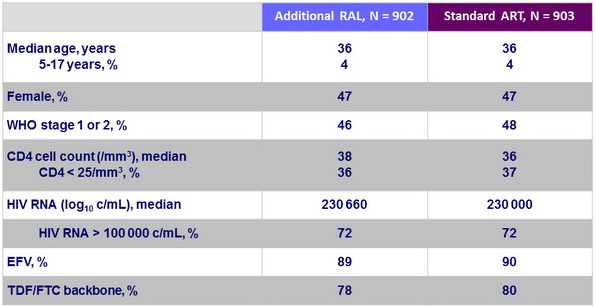
Baseline characteristics
Mortality (left) & HIV RNA < 50 copies/mL (95% CI) (right)
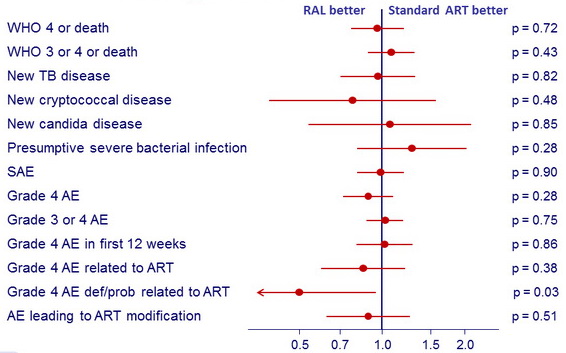
Secondary/other outcomes (HR, 95% CI)