Reynes J. HIV Clin Trials. 2011 Sep-Oct;12(5):255-67; AIDS Res Hum Retroviruses. 2013 Feb;29(2):256-65
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» 2 drugs vs 3 drugs
» NRTI-Sparing
» LPV/r + RAL vs LPV/r + FTC/TDF
Head-to-head comparative trials for first line ART since 2006
» 2 drugs vs 3 drugs
» NRTI-Sparing
» LPV/r + RAL vs LPV/r + FTC/TDF
Drugs
RAL, LPV/r, FTC/TDF, TDF, FTC
RAL, LPV/r, FTC/TDF, TDF, FTC
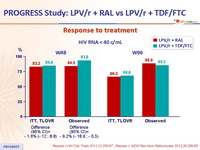
- Through 96 weeks, LPV/r + RAL demonstrated similar efficacy, safety and tolerability than the traditional triple combination of LPV/r + TDF/FTC
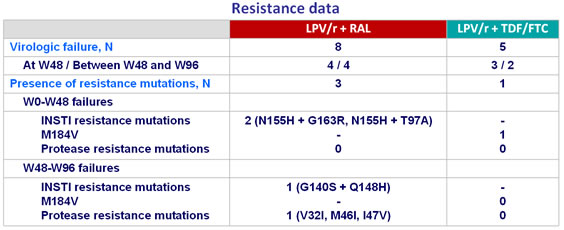
- Emergence of resistance mutations infrequent
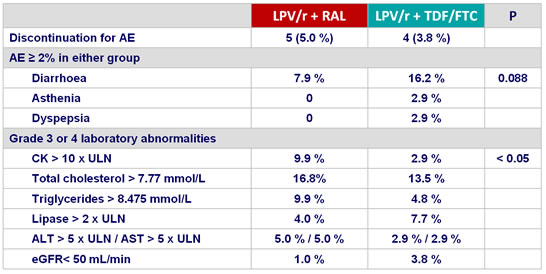
- Lipid changes more favourable with LPV/r + TDF/FTC
- Decrease of eGFR more pronounced with LPV/r + TDF/FTC
- No change in bone mineral density with LPV/r + RAL
- Limitations
- Sample size
- Low proportion of patients with baseline HIV-1 RNA > 100,000 c/mL
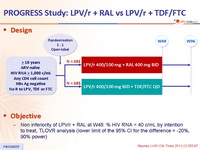
Design :
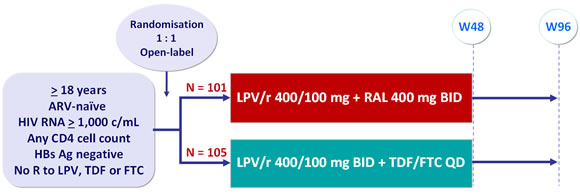
Objective :
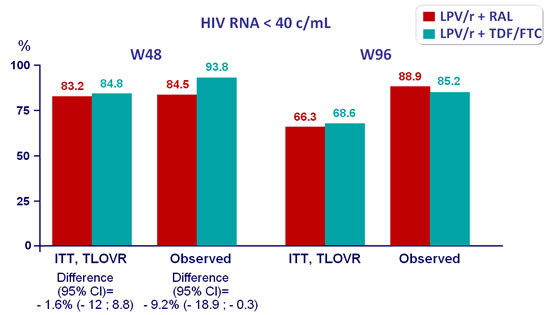
- Non inferiority of LPV/r + RAL at W48: % HIV RNA < 40 c/mL by intention to treat, TLOVR analysis (lower limit of the 95% CI for the difference = -20%, 90% power)
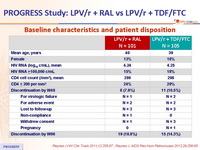
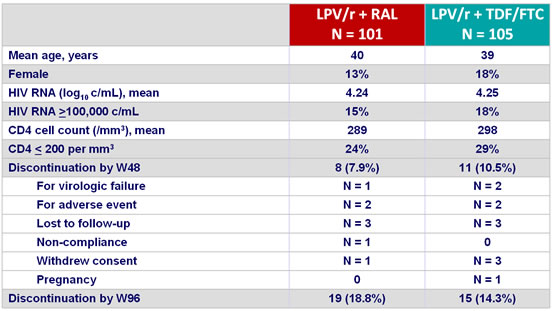
Baseline characteristics and patient disposition :
Response to treatment :
Protocol-defined criteria for genotype testing :
- At or after W8, in patients having achieved HIV-1 RNA < 40 c/mL, HIV-1 RNA ≥ 40 c/mL with confirmatory sample > 400 c/mL
- HIV-1 RNA increase > 0.5 log10 c/mL above study nadir and > 400 c/mL on 2 consecutive measurements
- Failure to achieve HIV-1 RNA < 400 c/mL at by week 24
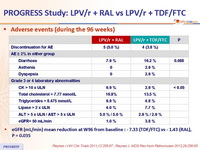
Adverse events (during the 96 weeks) :
- eGFR (mL/min) mean reduction at W96 from baseline : - 7.33 (TDF/FTC) vs - 1.43 (RAL), P = 0.035
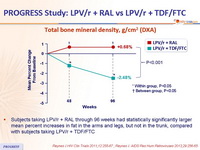
Total bone mineral density, g/cm2 (DXA) :
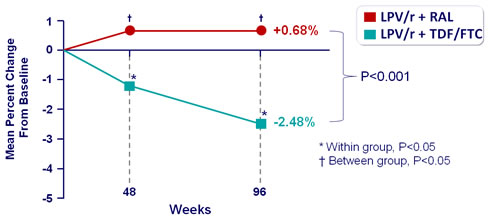
- Subjects taking LPV/r + RAL through 96 weeks had statistically significantly larger mean percent increases in fat in the arms and legs, but not in the trunk, compared with subjects taking LPV/r + TDF/FTC