Paton NI. Lancet HIV. 2015 Oct;2(10):e417-26
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» PI/r mono
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» PI/r mono
Drugs
PI/r mono
PI/r mono
- In patients who have achieved viral load suppression with combination treatment, a maintenance strategy of PI/r monotherapy, with reintroduction of combination treatment in the event of viral load rebound, was non-inferior to continuous combination treatment for preservation of future treatment options during 3–5 years
- Regular viral load monitoring and prompt reintroduction of combination treatment for rebound needed
- Absolute number of patients who lost future drug options with PI/r monotherapy was very low (only 1 patient with resistance to ATV)
- No change in overall clinical outcomes or frequency of toxic effects
- Much higher proportion of patients in the PI/r monotherapy group with viral rebound
- Rapid resuppression of viral load by reintroduction of combination treatment
- No adverse effect on CD4 change
- Protease inhibitor monotherapy is an acceptable alternative for long-term clinical management of HIV infection
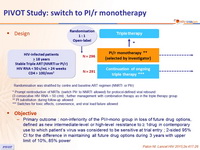
Design
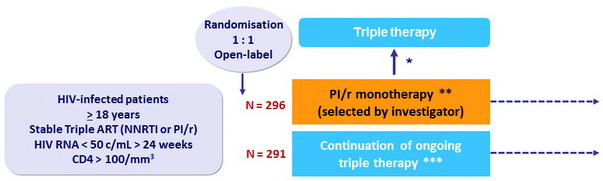
Randomisation was stratified by centre and baseline ART regimen (NNRTI or PI/r)
* Prompt reintroduction of NRTIs (switch PI/r to NNRTI allowed) for protocol-defined viral rebound
(3 consecutive HIV RNA > 50 c/ml) ; further management with combination therapy as in the triple therapy group
** PI substitution during follow-up allowed
*** Switches for toxic effects, convenience, and viral load failure allowed
Objective
- Primary outcome : non-inferiority of the PI/r-mono group in l oss of future drug options, defined as new intermediate-level or high-level resistance to ≥ 1drug in contemporary use to which patient's virus was considered to be sensitive at trial entry ; 2-sided 95% CI for the difference in maintaining all future drug options during 3 years with upper limit of 10%, 85% power
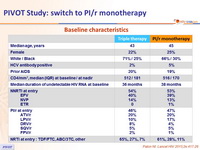
Baseline characteristics
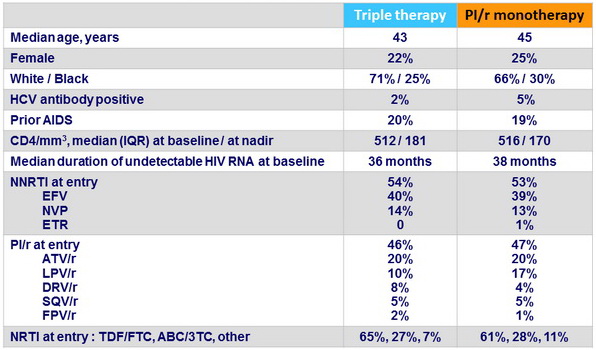
PI/r monotherapy group
- DRV/r : 80 %
- LPV/r : 14 %
- ATV/r : 6 %
- Saquinavir /r < 1 %
- 58% still on PI/r monotherapy at trial end (72% of follow-up time on monotherapy )
- Reasons for reintroduction of combination regimens
- 23% for protocol-defined confirmed viral rebound
- 4% for viral rebound not meeting protocol criteria
- 5% for toxic effects
- 7% for other or unknown reasons
Median duration of follow-up : 44 months
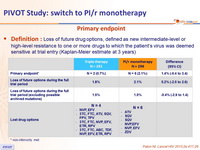
Primary endpoint
- Definition: Loss of future drug options, defined as new intermediate-level or high-level resistance to one or more drugs to which the patient's virus was deemed sensitive at trial entry (Kaplan-Meier estimate at 3 years)
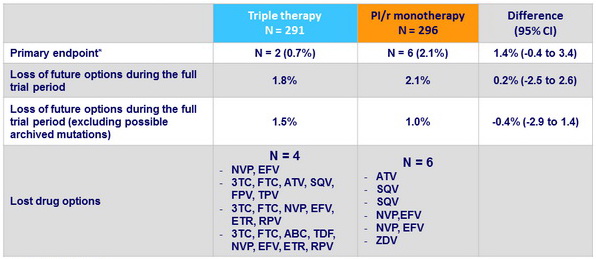
* non-inferiority met
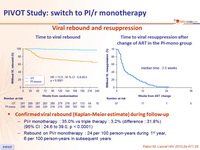
Viral rebound and resuppression
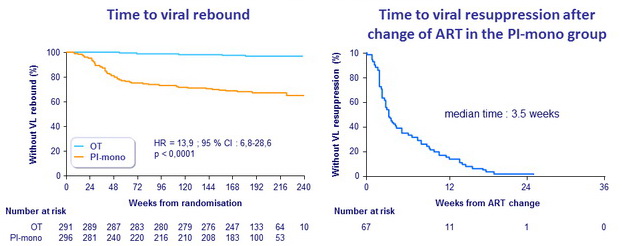
Confirmed viral rebound (Kaplan-Meier estimate) during follow-up
- PI/r monotherapy : 35.0% vs triple therapy : 3.2% (difference : 31.8%) (95% CI : 24.6 to 39.0, p < 0.0001)
- Rebound on PI/r monotherapy : 24 per 100 person-years during 1 st year, 6 per 100 person-years in subsequent years
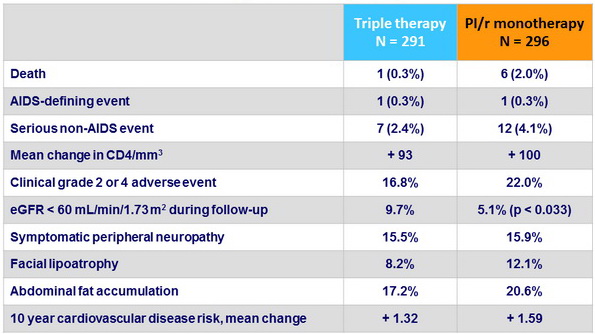
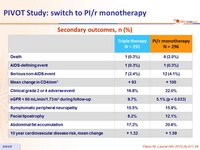
Secondary outcomes, n (%)