Pierone G. HIV Clin Trials. 2006 Sep-Oct;7(5):237-45
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
Drugs
LPV/r
LPV/r
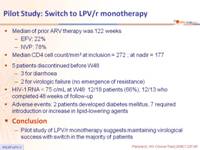
- Pilot study of LPV/r monotherapy suggests maintaining virological success with switch in the majority of patients
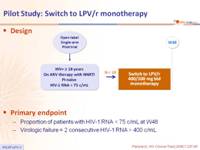
Design :

Primary endpoint :
- Proportion of patients with HIV-1 RNA < 75 c/mL at W48
- Virologic failure = 2 consecutive HIV-1 RNA > 400 c/mL
- Median of prior ARV therapy was 122 weeks
- EFV: 22%
- NVP: 78%
- Median CD4 cell count/mm3 at inclusion = 272 ; at nadir = 177
- 5 patients discontinued before W48
- 3 for diarrhoea
- 2 for virologic failure (no emergence of resistance)
- HIV-1 RNA < 75 c/mL at W48: 12/18 patients (66%), 12/13 who completed 48 weeks of follow-up
- Adverse events: 2 patients developed diabetes mellitus, 7 required introduction or increase in lipid-lowering agents