Cahn P. Lancet HIV, 2017;4:e486-94 ; Cahn P, JAIDS 2018;78:589-98
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» INSTI vs INSTI
» RAL 1200 QD vs 400 BID + FTC/TDF
Head-to-head comparative trials for first line ART since 2006
» INSTI vs INSTI
» RAL 1200 QD vs 400 BID + FTC/TDF
Drugs
RAL, FTC/TDF, TDF, FTC
RAL, FTC/TDF, TDF, FTC
- In HIV-1 treatment-naive patients, RAL1200 mg (two 600 mg reformulated tablets) QD has potent and durable efficacy comparable
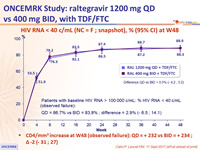
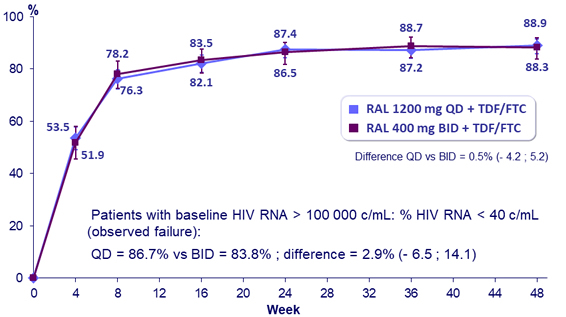
to RAL 400 mg BID each in combination with TDF/FTC:- Statistically non-inferior antiretroviral activity of RAL 1200 mg QD compared to RAL 400 mg BID, with 88.9% achieving HIV RNA < 40 c/mL at W48 and 81.5% at W96
- High and similar rates of virologic suppression, irrespective of baseline HIV RNA
- Low frequency of RAL resistance (0.75%) in both treatment groups
- Large increases in CD4 count (232 cells/mm3) comparable to RAL 400 mg BID (234 cells/mm3) at W48 (+ 262/mm3 at W96)
- RAL 1200 mg QD was generally well tolerated
- Overall safety profile similar to RAL 400 mg BID
- Reformulated once-daily raltegravir offers a new potent, well tolerated, and convenient option for initial treatment of HIV infection
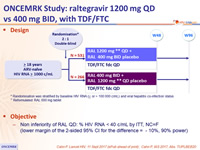
Design
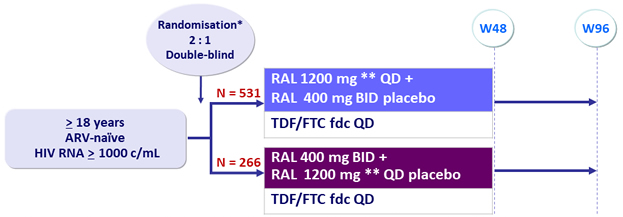
* Randomisation was stratified by baseline HIV RNA ( < or > 100 000 c/mL) and viral hepatitis co-infection status
** Reformulated RAL 600 mg tablet
Objective
- Non inferiority of RAL QD: % HIV RNA < 40 c/mL by ITT, NC=F (lower margin of the 2-sided 95% CI for the difference = - 10%, 90% power)
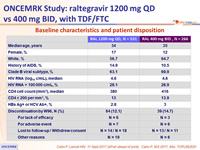
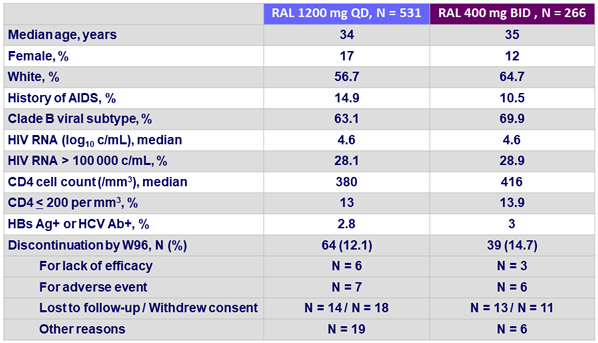
Baseline characteristics and patient disposition
HIV RNA < 40 c/mL (NC = F ; snapshot), % (95% CI) at W48
- CD4/mm3 increase at W48 (observed failure): QD = + 232 vs BID = + 234 ; Δ -2(- 31 ; 27)
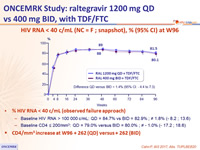
HIV RNA < 40 c/mL (NC = F ; snapshot), % (95% CI) at W96
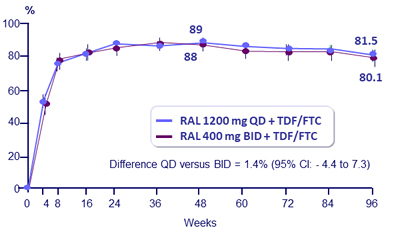
- % HIV RNA < 40 c/mL (observed failure approach)
- Baseline HIV RNA > 100 000 c/mL: QD = 84.7% vs BID = 82.9% ; ≠1.8% (- 8.2 ; 13.6)
- Baseline CD4 ≤ 200/mm3 : QD = 79.0% versus BID = 80.0% ; ≠- 1.0% (- 17.2 ; 18.6)
- CD4/mm3 increase at W96 + 262 (QD) versus + 262 (BID)
Virologic failure
- Non response: did not achieve HIV RNA < 40 c/ mL by W24
- Rebound: 2 consecutive measurements of HIV-1 RNA ≥ 40 c/mL at least 1 week apart after initial response of HIV RNA < 40 c/ mL
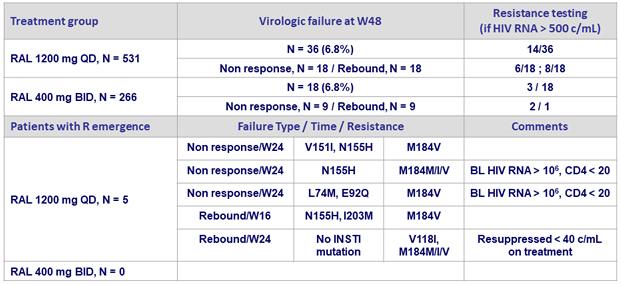
Resistance data at W96
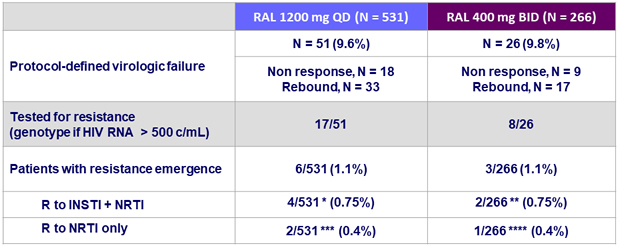
* RAL 1200 mg QD: R to INSTI = N155H (N = 1), V151I + N155H (N = 1), L74M, + E92Q (N = 1), N155H + I203M (N = 1) ; R to NRTI = M184V (N = 3), M184M/I/V (N = 1)
** RAL 400 mg BID: R to INSTI: T97A + I203M (N = 1) ; L74I + N155H + I203M (N = 1) ; R to NRTI: M184V (N = 1), M184V + K65R (N = 1)
*** Resistance to FTC
**** Resistance to FTC and TDF
Clinical adverse events at W48, %
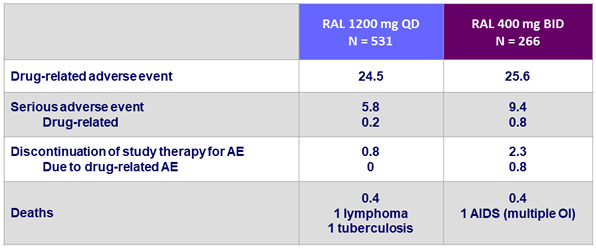
- Between W48 and W96, only 1 additional patient (in the QD group) discontinued study drug for adverse event (not drug-related)