Arribas JR. Lancet Infect Dis 2015; 2015; 15:785-92
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r + 3TC
» LPV/r + 3TC vs LPV/r + 2 NRTI
Switch studies in virologically suppressed patients
» Switch to PI/r + 3TC
» LPV/r + 3TC vs LPV/r + 2 NRTI
Drugs
LPV/r, 2 NRTI
LPV/r, 2 NRTI
- In virologically suppressed patients on a triple-drug antiretroviral regimen with LPV/r + 2NRTI, switching to LPV/r + 3TC or FTC demonstrated non-inferior efficacy and comparable safety to LPV/r + 2 NRTI, as maintenance therapy
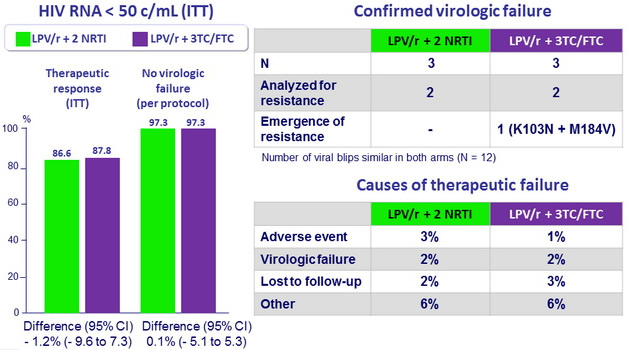
- Percentage of patients with protocol defined virological failure were very small and similar between arms
- Dual therapy with LPV/r + 3TC or FTC has the potential benefit of preserving future options, reducing the cost of antiretroviral therapy and minimizing potential long term toxicity
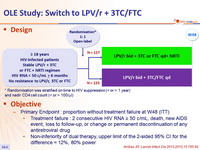
Design :
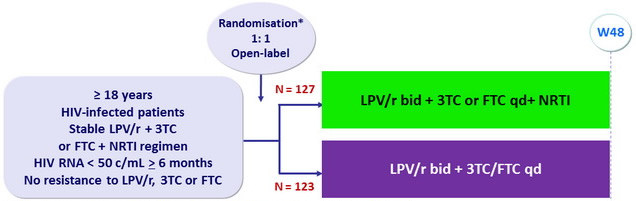
* Randomisation was stratified on time to HIV suppression (< or > 1 year)
and nadir CD4 cell count (< or > 100/ m l)
Objective :
- Primary Endpoint : proportion without treatment failure at W48 (ITT)
- Treatment failure : 2 consecutive HIV RNA ≥ 50 c/mL, death, new AIDS event, loss to follow-up, or change or permanent discontinuation of any antiretroviral drug
- Non-inferiority of dual therapy, upper limit of the 2-sided 95% CI for the difference = 12%, 80% power
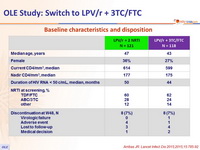
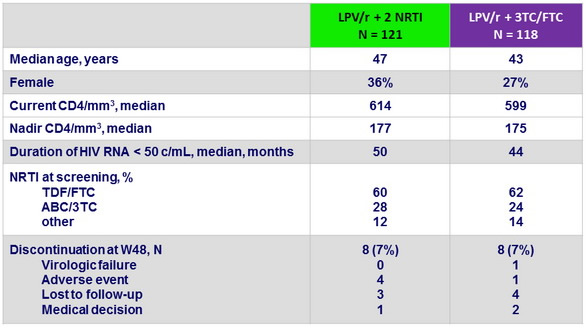
Baseline characteristics and patient disposition :
Efficacy results at W48
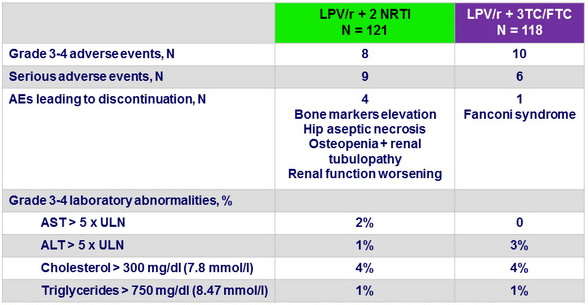
Safety at W48
Small but significant increase of eGFR (MDRD), total and LDL cholesterol in the dual treatment group at 48 weeks compared with the triple treatment group