Pulido F. AIDS. 2008 Jan 11;22(2):F1-9 ; Arribas JR. J Acquir Immune Defic Syndr. 2009 Jun 1;51(2):147-52
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» LPV/r mono vs LPV/r + 2 NRTI
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» LPV/r mono vs LPV/r + 2 NRTI
Drugs
PI/r mono, LPV/r
PI/r mono, LPV/r
- From W48 data
- In patients with virologic suppression for more than 6 months on LPV/r + 2 NRTIs, LPV/r monotherapy followed by re-introduction of the NRTIs as required is a therapeutic strategy as effective as continuing triple therapy: non inferiority of monotherapy was demonstrated
- The vast majority of patients with loss of virologic suppression on LPV/r monotherapy had no evidence of resistance mutations in the protease gene and were able to resupress and maintain virologic suppression after resumption of baseline NRTIs
- From W96 data
- The 96 weeks results support the efficacy and safety of the LPV/r monotherapy strategy
- Although episodes of low-level viremia were more frequent in the monotherapy group, there was not an increased risk of resistance development and most of these patients could be virologically resupressed with addition of NRTIs
- The toxicity of the LPV/r monotherapy regimen was lower than the toxicity of the triple regimen
Design :
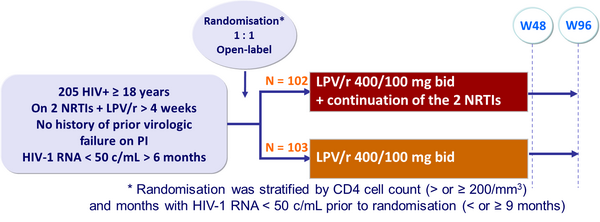
Objective :
- Non inferiority of the monotherapy group in the proportion of patients with therapeutic failure at W48 (per-protocol analysis) ; upper limit of the 95% CI for the difference = 12%, 80% power
- Therapeutic failure: 2 consecutive HIV-1 RNA > 500 c/mL (if no resistance at failure and successful viral suppression after reintroduction of 2 NRTIs, not considered as failure) ; change of randomised therapy ; treatment discontinuation ; loss to follow-up
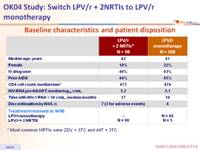
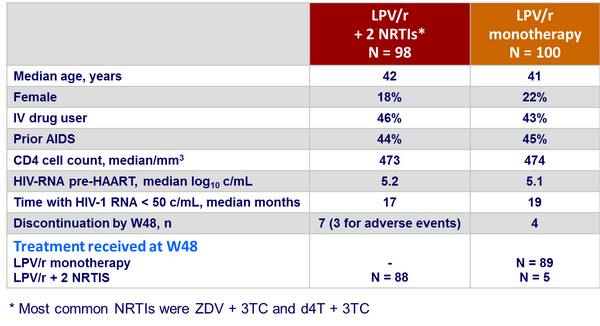
Baseline characteristics and patient disposition :
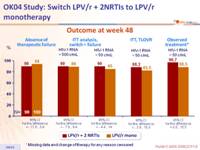
Outcome at week 48 :
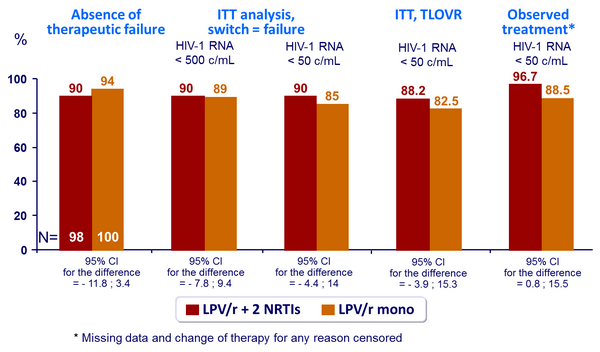
Response to treatment at week 48 :
- Therapeutic failures
- 6 in the monotherapy group
- 3 were lost to follow-up
- 1 changed randomised therapy without loss of virologic suppression
- 1 lost virologic suppression and developed PI resistance
- 1 lost virologic suppression and failed to achieve suppression after reintroduction of NRTIs
- 10 in the triple therapy arm
- 4 were lost to follow-up
- 3 had confirmed loss of virologic suppression
- 3 discontinued randomised treatment due to adverse events
- 6 in the monotherapy group
- Loss of virologic suppression at W48
- 3 in the triple therapy group
- 6 in the monotherapy group: of these 6, 4 resumed baseline NRTIs and regained virologic suppression, 1 had LPV/r resistance, and 1 did not achieve virologic suppression after resuming baseline NRTIs
Safety, Adverse events, Resistance, Adherence (W48) :
- Study drug-related adverse events of at least moderate severity
- 3 in the triple therapy group (diarrhoea, N = 2, insomnia, N = 1), leading to treatment discontinuation
- None in the monotherapy group (p = 0.08)
- No statistical significant changes from baseline in fasting total cholesterol, HDL cholesterol or triglycerides in both groups
- Genotypic analysis in patients with HIV-1 RNA > 500 c/mL
- 3 in the triple therapy group:
- PI resistance, N = 1 (mutations 54V, 63P, 71V and 82A)
- NRTI resistance, N = 1
- 11 in the monotherapy group:
- PI resistance, N = 2 (mutations 10F and 46I in 1 ; mutations 54V, 77I and 82A in 1)
- NRTI resistance, N = 1
- 3 in the triple therapy group:
- No difference in adherence between the 2 groups
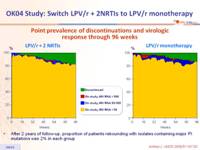
Outcome at week 96 :
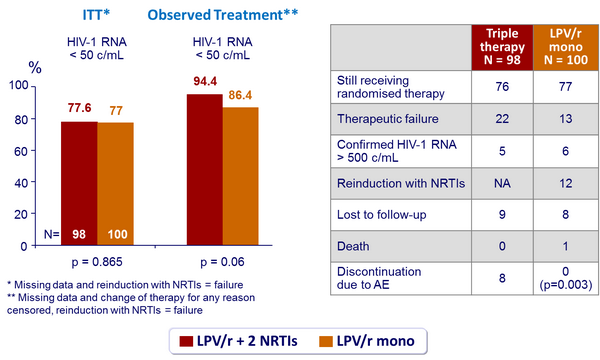
Point prevalence of discontinuations and virologic response through 96 weeks :
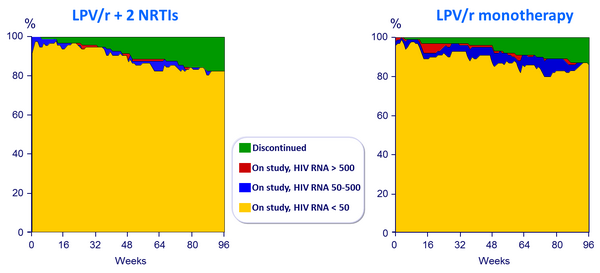
- After 2 years of follow-up, proportion of patients rebounding with isolates containing major PI mutations was 2% in each group