Arribas JR. J Acquir Immune Defic Syndr. 2005 Nov 1;40(3):280-7
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» LPV/r mono vs LPV/r + 2 NRTI
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» LPV/r mono vs LPV/r + 2 NRTI
Drugs
PI/r mono, LPV/r, 2 NRTI
PI/r mono, LPV/r, 2 NRTI
- This pilot study provides preliminary evidence suggesting that in patients suppressed on a triple therapy with LPV/r, continuation with LPV/r monotherapy can maintain HIV suppression in a large proportion of patients
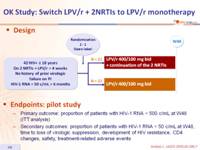
Design :
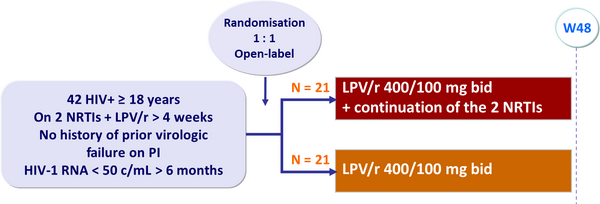
Endpoints: pilot study :
- Primary outcome: proportion of patients with HIV-1 RNA < 500 c/mL at W48 (ITT analysis)
- Secondary outcomes: proportion of patients with HIV-1 RNA < 50 c/mL at W48, time to loss of virologic suppression, development of HIV resistance, CD4 changes, safety, treatment-related adverse events
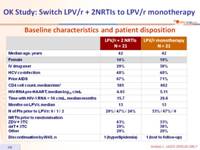
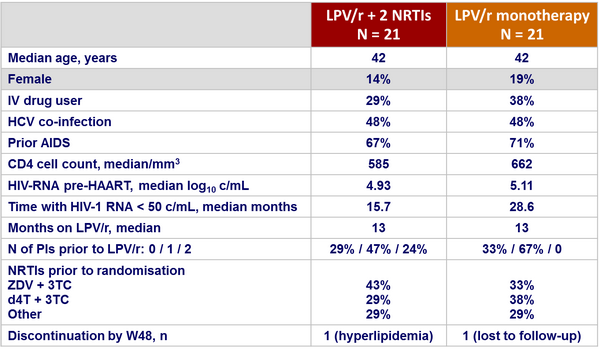
Baseline characteristics and patient disposition :
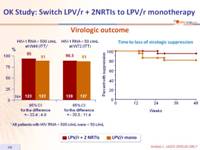
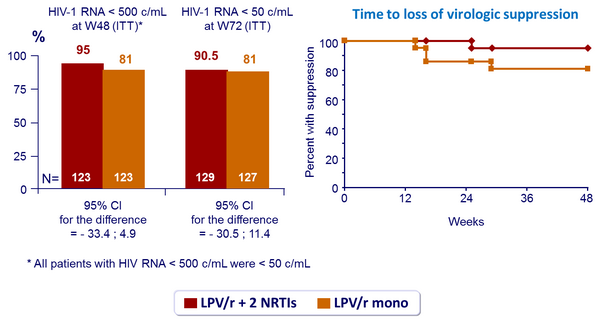
Virologic outcome :
Other outcomes :
- 4 blips in the monotherapy group vs 1 in the triple therapy group
- No significant changes in CD4 cell count in any group at W48
- Median adherence was 96% in the triple therapy group and 86% in the monotherapy group (p = 0.09)
- Loss of virologic suppression was associated with shorter time of HIV-1 RNA < 50 c/mL prior to monotherapy (median of 40 weeks vs 132 weeks in patients with maintenance of virologic suppression ; p = 0.02)
- Patients with loss of virologic suppression on LPV/r monotherapy were safely reinduced with prior NRTIs and achieved HIV-1 RNA < 50 c/mL
- Grade 3 hypertriglyceridemia occurred in 3 patients in the triple therapy arm vs none in the monotherapy arm. One patient in each arm had grade 3 hypercholesterolemia