Gutmann C. AIDS. 2010 Sep 24;24(15):2347-54
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» LPV/r mono
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» LPV/r mono
Drugs
PI/r mono, LPV/r
PI/r mono, LPV/r
- Maintenance of HIV treatment with LPV/r monotherapy should not be recommended as a standard strategy ; this is particularly evident in patients with a CD4 cell count < 200/mm3 at nadir
- The proportion of patients with detectable HIV-1 RNA in CSF was not only significantly higher on LPV/r monotherapy than on continued combination therapy (32% vs 0% ; p = 0.01), but the difference appears biologically (CSF inflammation) and clinically (acute symptoms) relevant
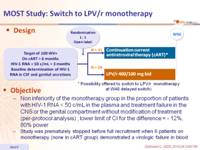
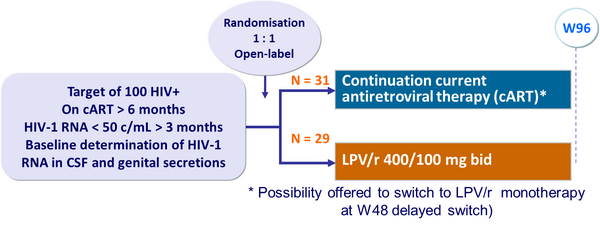
Design :
Objective :
- Non inferiority of the monotherapy group in the proportion of patients with HIV-1 RNA < 50 c/mL in the plasma and treatment failure in the CNS or the genital compartment without modification of treatment (per-protocol analysis) ; lower limit of CI for the difference = - 12%, 80% power
- Study was prematurely stopped before full recruitment when 6 patients on monotherapy (none in cART group) demonstrated a virologic failure in blood
Baseline characteristics :
Outcome :
- Median follow-up: 48 weeks
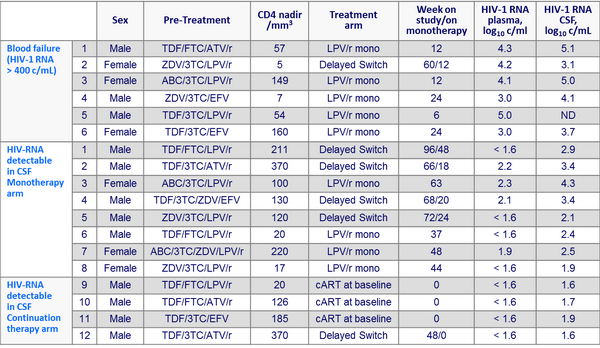
- Virologic failure (2 consecutive plasma HIV-1 RNA > 400 c/mL) occurred in 6/29 patients in the LPV/r monotherapy group, after a median of 12 weeks, vs 0/31 in the continued antiretroviral therapy group
- In these 6 failures, the median duration of HIV-1 RNA < 50 c/mL was 50 months ; 5/6 patients had clinical symptoms at the time of failure, all symptoms resolving after treatment switch ; all 6 patients had a nadir CD4 cell count < 200/mm3
- CSF was examined in 45 patients at study termination (25 on LPV/r monotherapy and plasma HIV-1 RNA < 400 c/mL, 5 failing monotherapy and 15 continuing prior ARV therapy with plasma HIV-1 RNA < 50 c/mL) CSF HIV-1 RNA was > 40 c/mL
- 8/25 patients on monotherapy
- none of the 15 patients still on continued treatment (p = 0.01)
- No marked elevation of HIV-1 RNA in the genital secretions
Patients with treatment failure in blood or detection of elevated HIV-1 RNA in CSF :