Delfraissy JF. AIDS. 2008 Jan 30;22(3):385-93
Type of ARV Trial
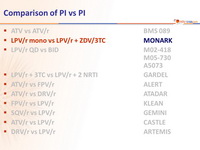
Head-to-head comparative trials for first line ART since 2006
» PI vs PI
» LPV/r mono vs LPV/r + ZDV/3TC
Head-to-head comparative trials for first line ART since 2006
» PI vs PI
» LPV/r mono vs LPV/r + ZDV/3TC
Drugs
PI/r mono, LPV/r, ZDV/3TC, 3TC, ZDV
PI/r mono, LPV/r, ZDV/3TC, 3TC, ZDV
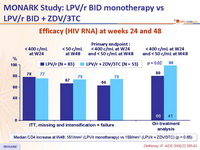
- In antiretroviral-naïve patients, LPV/r monotherapy demonstrates lower rates of virologic suppression as compared with LPV/r + ZDV/3TC
- LPV/r monotherapy should not be offered for first-line antiretroviral therapy
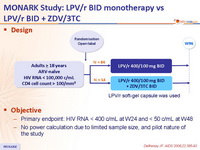
Design :
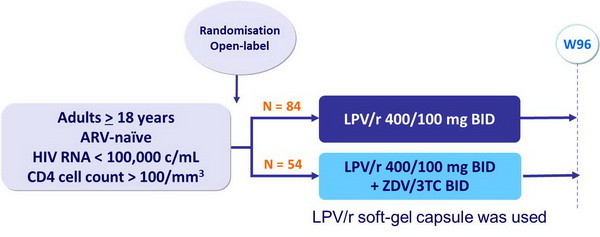
Objective :
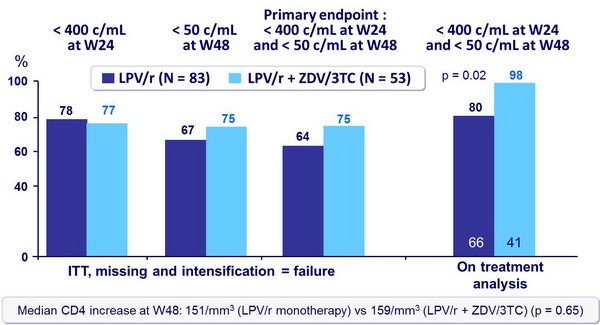
- Primary endpoint: HIV RNA < 400 c/mL at W24 and < 50 c/mL at W48
- No power calculation due to limited sample size, and pilot nature of the study
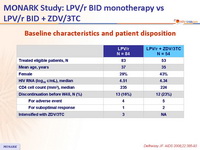
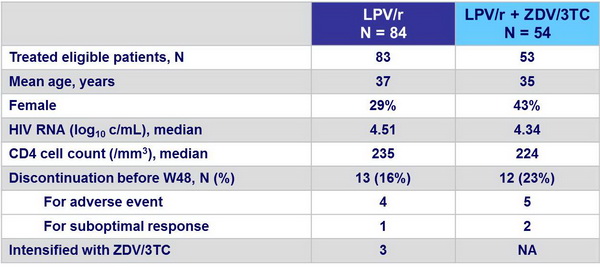
Baseline characteristics and patient disposition :
Efficacy (HIV RNA) at weeks 24 and 48 :
Resistance, safety and tolerability :
- 24/136 patients qualified for resistance testing (rebound of HIV RNA > 500 c/mL): 21/83 in the LPV/r monotherapy group and 3/53 in the LPV/r + ZDV/3TC group
- PI-associated resistance mutations emerged in 3/21 patients on LPV/r monotherapy (L76V, M46I)
- Serious adverse event: 12% LPV/r mono vs 8% LPV/r + ZDV/3TC
- Similar frequency of clinical adverse events (mainly diarrhoea) and laboratory abnormalities (transaminases elevations) of at least moderate severity in the 2 groups