Stellbrink HJ. AIDS 2016; 30:1229-38
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» 2 drugs vs 3 drugs
» NRTI-Sparing
» DRV/r + MVC vs DRV/r + FTC/TDF
Head-to-head comparative trials for first line ART since 2006
» 2 drugs vs 3 drugs
» NRTI-Sparing
» DRV/r + MVC vs DRV/r + FTC/TDF
Drugs
MVC, DRV/r, FTC/TDF, TDF, FTC
MVC, DRV/r, FTC/TDF, TDF, FTC
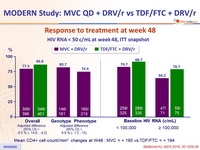
- MVC 150 mg QD + DRV/r QD was statistically inferior to TDF/FTC
+ DRV/r QD in antiretroviral-naïve subjects over 48 weeks - Lower rate of virologic suppression
- IDMC recommended study termination
- The majority of failure had HIV RNA < 400 c/ mL
- There was no treatment-emergent resistance in either arm
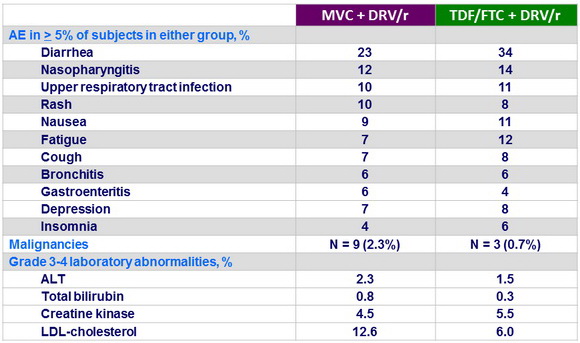
- Safety was comparable
- MVC 150 mg QD in dual therapy with DRV/r QD cannot be recommended as first-line antiretroviral therapy
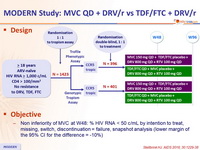
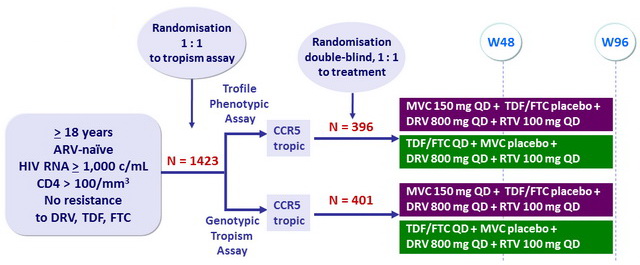
Design :
Objective :
- Non inferiority of MVC at W48: % HIV RNA < 50 c/mL by intention to treat, missing, switch, discontinuation = failure, snapshot analysis (lower margin of the 95% CI for the difference = -10%)
Baseline characteristics and patient disposition
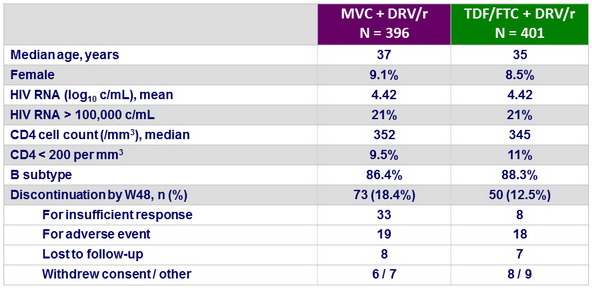
Study was terminated early upon recommendation of IDMC
Response to treatment at week 48
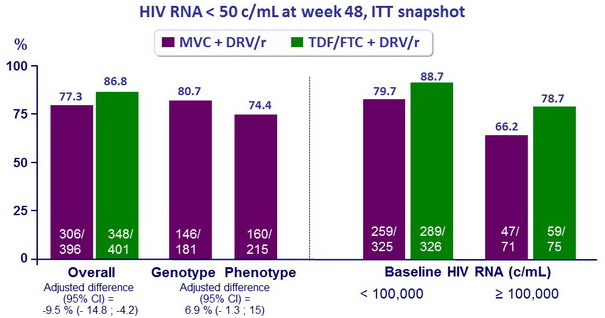
Mean CD4+ cell count//mm 3 changes at W48 : MVC = + 195 vs TDF/FTC = + 194
Protocol-defined treatment failure (PDTF) criteria
- Decrease in plasma HIV RNA < 1 log10 from baseline after W4, unless plasma HIV RNA is < 50 c/mL, or
- Plasma HIV RNA > 1 log10 c/mL above the nadir value after W4, or
- Plasma HIV RNA ≥ 50 c/mL at any time after W24, or
- Plasma HIV RNA ≥ 50 c/mL after suppression to < 50 c/mL on 2 consecutive visits, or
- Decrease in plasma HIV RNA < 2 log10 c/mL from baseline on or after W12, unless plasma HIV RNA is < 50 c/mL (amendment 2), and < 400 c/mL (amendment 3)
- All PDTFs required confirmation within 28 days of the initial event
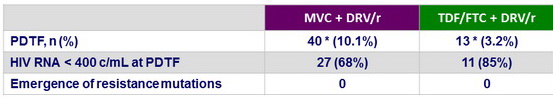
* 3 in each group had HIV RNA < 400 c/ mL and showed a response at W48
Treatment-emergent adverse events at week 48