Morales-Ramirez JO. CROI 2014, Abs. 92LB ; Gatell JM. HIV Drug Therapy 2014, Abs. O434
Type of ARV Trial
Phase 2 of new ARVs
» Doravirine (non nucleoside reverse transcriptase inhibitor)
» DOR + FTC/TDF vs EFV/FTC/TDF
Phase 2 of new ARVs
» Doravirine (non nucleoside reverse transcriptase inhibitor)
» DOR + FTC/TDF vs EFV/FTC/TDF
Drugs
DOR, EFV, FTC/TDF, TDF, FTC
DOR, EFV, FTC/TDF, TDF, FTC
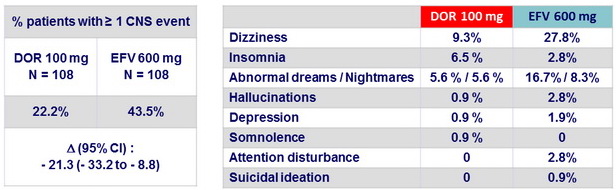
- In antiretroviral-naïve, HIV-1 infected subjects, DOR 100 mg qd + TDF/FTC had a lower rate of treatment-emergent CNS events by week 8 than EFV + TDF/FTC
- DOR 25 to 200 mg qd for 48 weeks
- had simialr virologic and immunologic efficacy to EFV
- with low rate of resistance mutation development
- and good safety and tolerability profile
- DOR 100 mg qd dose was selected for further development
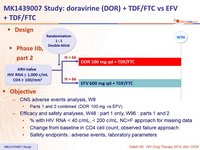
Design
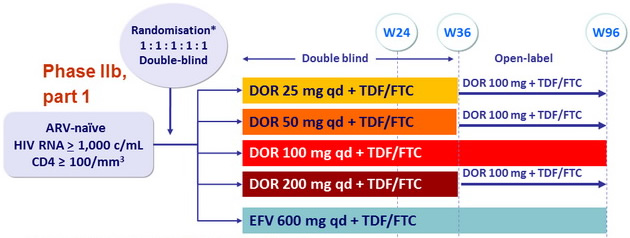
* Randomisation stratified on HIV RNA (> or ≤ 100,000 c/mL)
Objective
- Primary endpoints
- % HIV-1 RNA < 40 c/mL at W24 (estimation comparisons for DOR dose selection), ITT, NC=F
- Safety : general at W24, pre-specified CNS AEs by W8 and W24
Design
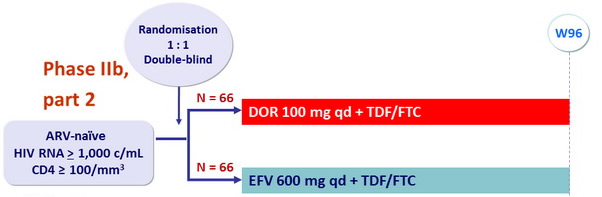
Objective
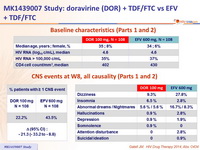
- CNS adverse events analysis, W8
- Parts 1 and 2 combined (DOR 100 mg vs EFV)
- Efficacy and safety analyses, W48 : part 1 only, W96 : parts 1 and 2
- % with HIV RNA < 40 c/ mL, < 200 c/ mL, NC=F approach for missing data
- Change from baseline in CD4 cell count, observed failure approach
- Safety endpoints : adverse events, laboratory parameters
Baseline characteristics and patient disposition (Part 1)
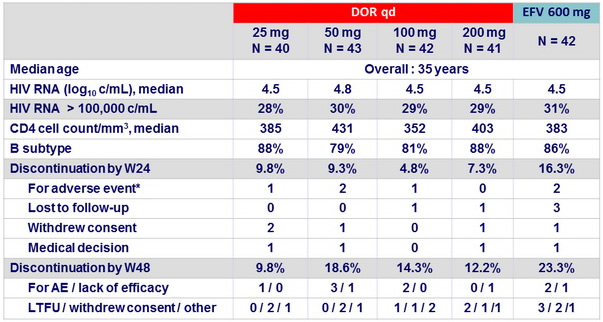
* DOR 25 mg : stupor (n = 1), DOR 50 mg: abdominal pain/nausea/insomnia (n = 1), sleep disorder (n = 1), DOR 100 mg : hallucinations
(n = 1), EFV : right-sided dysesthesia (n = 1), hallucinations (n = 1)
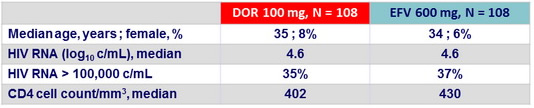
Baseline characteristics (Parts 1 and 2)
CNS events at W8, all causality (Parts 1 and 2)
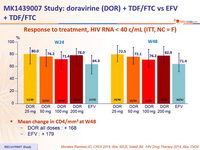
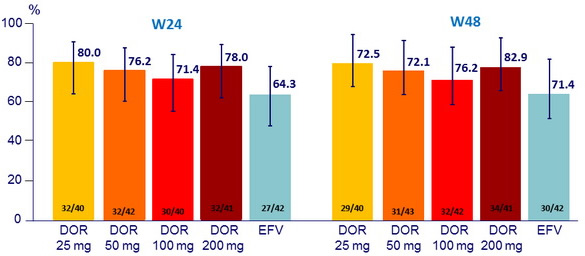
Response to treatment, HIV RNA < 40 c/ mL (ITT, NC = F)
Mean change in CD4/mm 3 at W48
- DOR all doses : + 168
- EFV : + 179
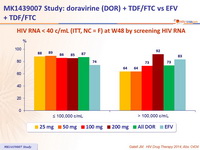
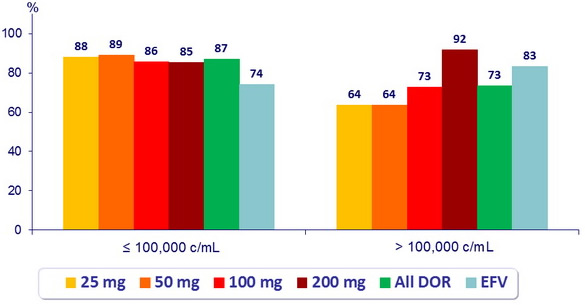
HIV RNA < 40 c/ mL (ITT, NC = F) at W48 by screening HIV RNA
Virologic failure definition
- Non-response : HIV RNA never < 40 c/mL by Week 24, or
- Rebound : after initial response of HIV RNA < 40 c/mL, 2 consecutive HIV RNA ≥ 40 c/mL at least 1 week apart, at or after Week 24
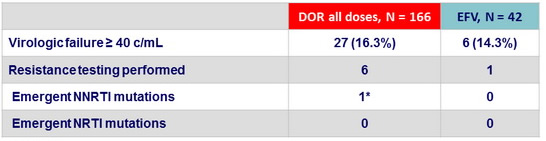
Criteria for resistance testing
- HIV RNA > 500 c/mL
Resistance data at virologic failure, W48
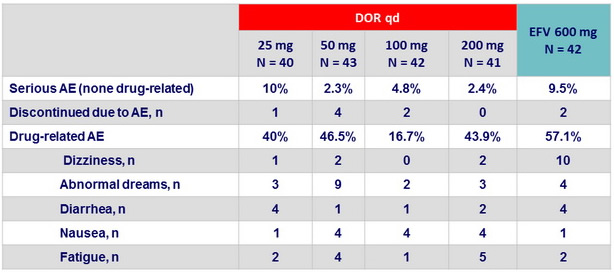
Clinical adverse events at W48 (Part 1)
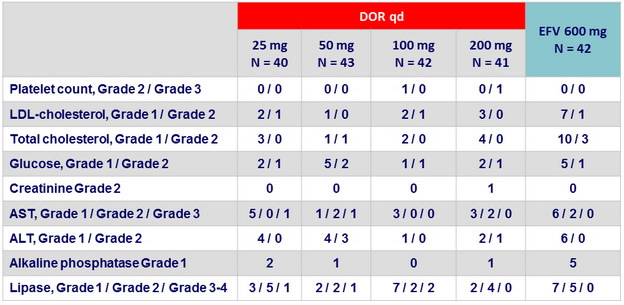
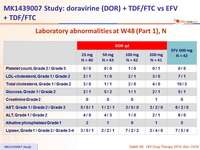
Laboratory abnormalities at W48 (Part 1 ), N