Pett SL. Clin Infect Dis 2016;63:122-32
Type of ARV Trial
Switch studies in virologically suppressed patients
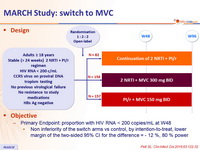
» Switch to MVC
» MVC + 2 NRTI vs MVC + PI/r vs PI/r + 2 NRTI
Switch studies in virologically suppressed patients
» Switch to MVC
» MVC + 2 NRTI vs MVC + PI/r vs PI/r + 2 NRTI
Drugs
MVC, ATV/r, LPV/r, 2 NRTI
MVC, ATV/r, LPV/r, 2 NRTI
- This large international randomised study demonstrates that MVC with a 2-N(t)RTI backbone, in those with R5-tropic virus determined by genotypic tropism testing, is a switch/simplification option for patients virologicaly suppressed on PI/r + N(t)RTI regimens
- MVC was safe and well tolerated, with favorable impact on lipids and neutral effects on renal function over 48 weeks
- These data support MVC as a switch option for ritonavir-boosted PIs when partnered with a 2-N(t)RTI backbone, but not as part of N(t)RTI-sparing regimens comprising MVC with PI/r
Design
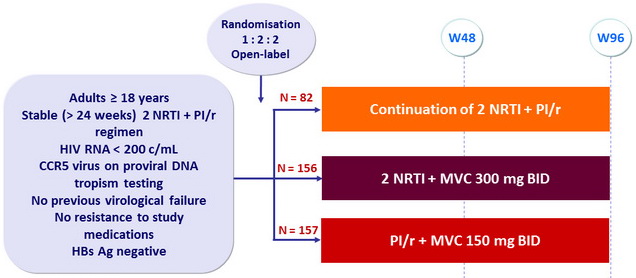
Objective
- Primary Endpoint: proportion with HIV RNA < 200 copies/mL at W48
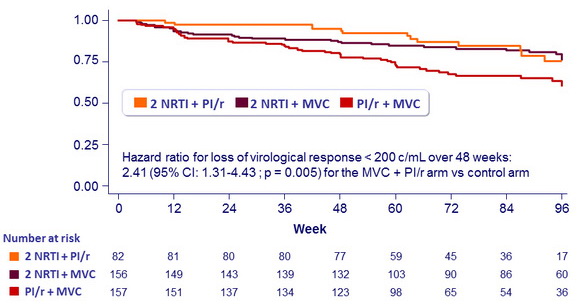
- Non inferiority of the switch arms vs control, by intention-to-treat, lower margin of the two-sided 95% CI for the difference = - 12 %, 80 % power
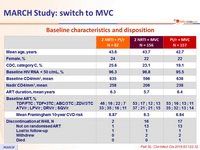
Baseline characteristics and disposition
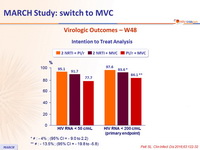
Virologic Outcomes – W48
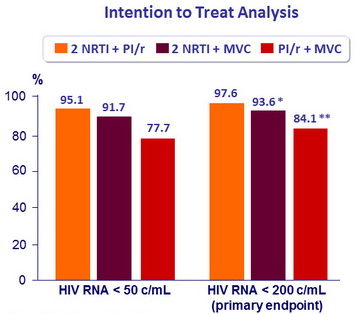
* ≠: - 4% ; (95% CI = - 9.0 to 2.2)
** ≠: - 13.5% ; (95% CI = - 19.8 to -5.8)
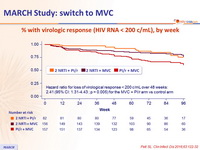
% with virologic response (HIV RNA < 200 c/mL), by week
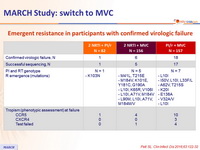
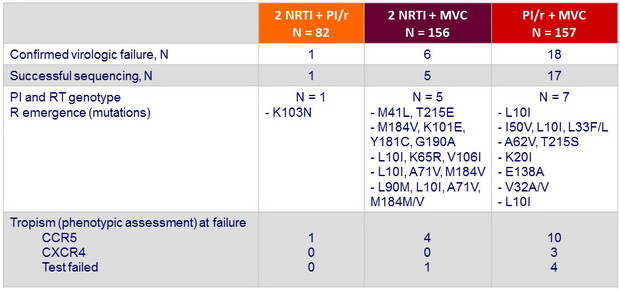
Emergent resistance in participants with confirmed virologic failure
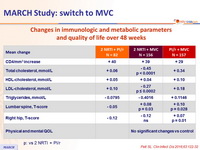
Changes in immunologic and metabolic parameters and quality of life over 48 weeks
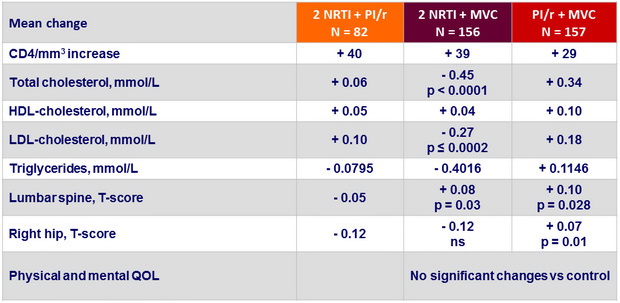
p : vs 2 NRTI + PI/r
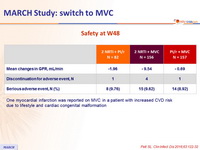
Safety at W48
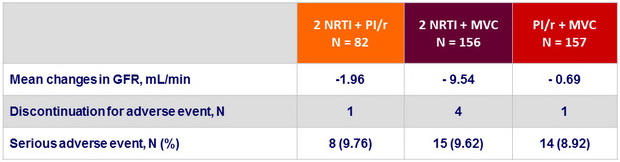
-
One myocardial infarction was reported on MVC in a patient with increased CVD risk due to lifestyle and cardiac congenital malformation