Gathe J. J Acquir Immune Defic Syndr. 2009 Apr 15;50(5):474-81
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» PI vs PI
» LPV/r QD + FTC + TDF vs LPV/r BID + FTC + TDF
Head-to-head comparative trials for first line ART since 2006
» PI vs PI
» LPV/r QD + FTC + TDF vs LPV/r BID + FTC + TDF
Drugs
LPV/r, FTC/TDF, TDF, FTC
LPV/r, FTC/TDF, TDF, FTC
- In antiretroviral-naïve adults, LPV/r QD was virologically non inferior at W48 to LPV/r BID, when administered in combination with TDF and FTC
- During the first 48 weeks of therapy, there were no significant differences in the safety or tolerability of QD vs BID LPV/r
- This study used LPV/r tablets, and did not show differences in rate of diarrhoea between QD and BID dosing
- In subgroups with high baseline HIV RNA and/or low CD4 count, efficacy of LPV/r QD and BID was similar
- Absence of resistance emergence to LPV/r or TDF, in either groups
- Limited and similar lipid impact of both LPV/r dosing
- Patient preference of the tablet over the soft-gel capsule
- Results support the use of LPV/r QD in combination with TDF and FTC in antiretroviral-naive patients
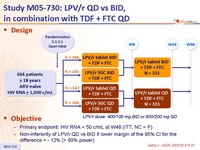
Design :
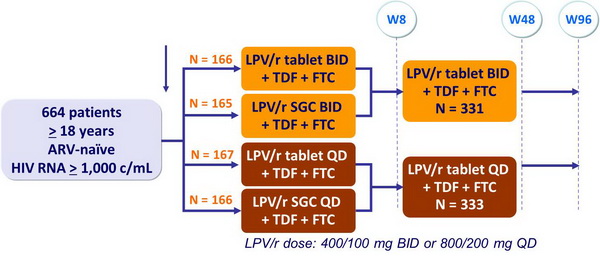
Objective :
- Primary endpoint: HIV RNA < 50 c/mL at W48 (ITT, NC = F)
- Non-inferiority of LPV/r QD vs BID if lower margin of the 95% CI for the difference = - 12% (> 90% power)
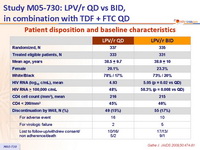
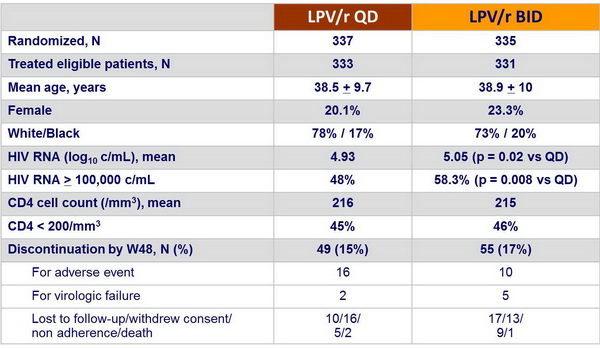
Patient disposition and baseline characteristics :
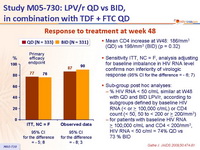
Response to treatment at week 48 :
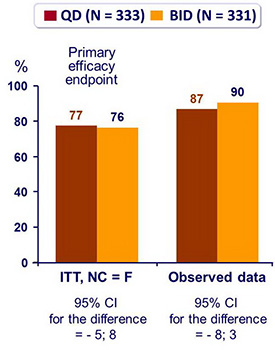
- Mean CD4 increase at W48: 186/mm3 (QD) vs 198/mm3 (BID) (p = 0.32)
- Sensitivity ITT, NC = F, analysis adjusting for baseline imbalance in HIV RNA level confirms non inferiority of virologic response (95% CI for the difference = - 6; 7)
- Sub-group post hoc analyses:
- % HIV RNA < 50 c/mL similar at W48 with QD and BID LPV/r, according to subgroups defined by baseline HIV RNA (< or > 100,000 c/mL) or CD4 count (< 50, 50 to < 200 or > 200/mm3)
- for patients with baseline HIV RNA > 100,000 c/mL and CD4 < 200/mm3, HIV RNA < 50 c/ml = 74% QD vs 73 % BID
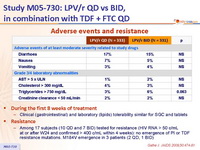
Adverse events and resistance :
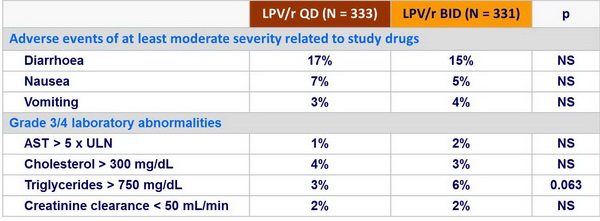
- During the first 8 weeks of treatment
- Clinical (gastrointestinal) and laboratory (lipids) tolerability similar for SGC and tablets
- Resistance
- Among 17 subjects (10 QD and 7 BID) tested for resistance (HIV RNA > 50 c/mL at or after W24 and confirmed > 400 c/mL within 4 weeks): no emergence of PI or TDF resistance mutations. M184V emergence in 3 patients (2 QD, 1 BID)