Cameron DW. J Infect Dis. 2008 Jul 15;198(2):234-40
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» LPV/r mono vs EFV + ZDV/3TC
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» LPV/r mono vs EFV + ZDV/3TC
Drugs
PI/r mono, LPV/r, EFV, ZDV/3TC, 3TC, ZDV
PI/r mono, LPV/r, EFV, ZDV/3TC, 3TC, ZDV
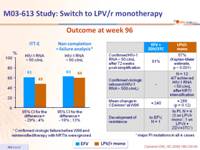
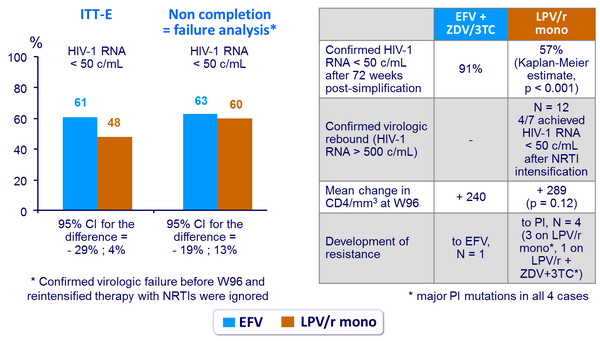
- LPV/r monotherapy was less effective than EFV + ZDV/3TC in maintaining virologic suppression: time to confirmed virologic rebound was shorter with LPV/r monotherapy
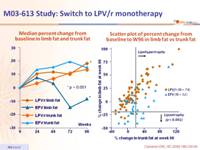
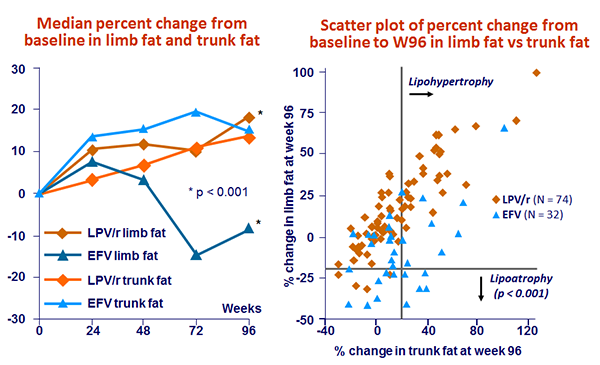
- Lipoatrophy was significantly lower in the LPV/r monotherapy group
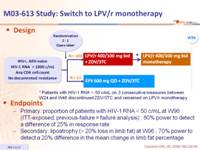
Design :
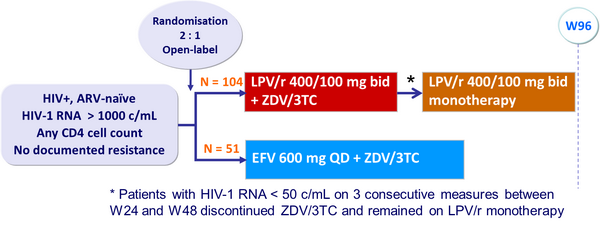
Endpoint :
- Primary: proportion of patients with HIV-1 RNA < 50 c/mL at W96 (ITT-exposed, previous-failure = failure analysis) ; 80% power to detect a difference of 25% in response rate
- Secondary: lipoatrophy (> 20% loss in limb fat) at W96 ; 70% power to detect a 20% difference in the mean change in limb fat percentage
Baseline characteristics and patient disposition :
- 79% of patients were male
- 65% were white
- Mean age was 38 years
- Mean baseline HIV-1 RNA was 4.9 log10 c/mL
- Patients in the LPV/r group had a higher mean baseline HIV-1 RNA and a higher mean age
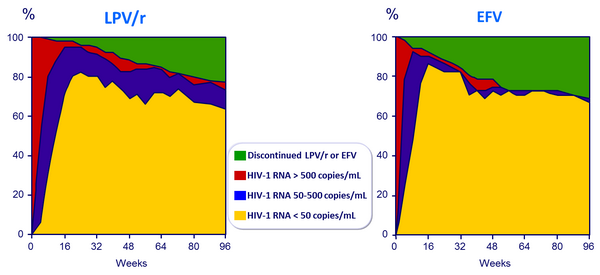
- 112 patients (57% in the LPV/r group and 69% in the EFV group) completed their assigned treatment regimen out to week 96
- In the LPV/r group, after a median of 24 weeks, 92 patients (88%) simplified to LPV/r monotherapy
Outcome at week 96 :
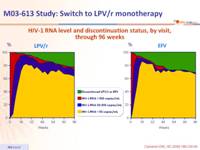
HIV-1 RNA level and discontinuation status, by visit, through 96 weeks :
Adverse events :
- Most common (frequency > 5%) moderate or severe adverse events related to treatment
- LPV/r monotherapy group
- Diarhoea: 15%
- Nausea: 14%
- EFV group
- Asthenia: 12%
- Dizziness: 12%
- Insomnia: 12%
- Rash: 10%
- Depression: 6%
- LPV/r monotherapy group
- Most frequent grade 3 or 4 laboratory abnormalities
- LPV/r monotherapy group
- Total cholesterol > 7.8 mmol/L: 12% ; Triglycerides > 8.5 mmol/L: 7%
- Amylase > 2 ULN: 6%
- EFV group
- Amylase > 5 ULN: 10%
- ALAT > 5 ULN: 6%
- LPV/r monotherapy group