Johnson MA. J Acquir Immune Defic Syndr. 2006 Oct 1;43(2):153-60
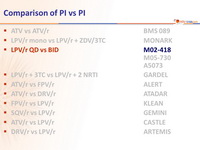
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» PI vs PI
» LPV/r QD + FTC + TDF vs LPV/r BID + FTC + TDF
Head-to-head comparative trials for first line ART since 2006
» PI vs PI
» LPV/r QD + FTC + TDF vs LPV/r BID + FTC + TDF
Drugs
LPV/r, FTC/TDF, TDF, FTC
LPV/r, FTC/TDF, TDF, FTC
- In previously untreated HIV-1 infected adults, LPV/r soft-gel capsule 800/200 mg QD was non inferior to LPV/r 400/100 mg BID, in combination with TDF + FTC QD
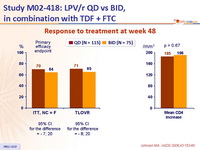
- Virologic response rate at W48 (HIV RNA < 50 c/mL) was 70% in the QD group and 64% in the BID group
- Immunologic recovery was similar in the 2 treatment arms
- There were greater number of discontinuations for adverse events (primarily gastrointestinal) and a significantly higher rate of diarrhoea in the QD group
- No significant differences in lipid changes was seen between the 2 groups
- Most pronounced lipid effect was triglyceride elevation
- Lipid increases were less than observed with LPV/r + thymidine analogues
- Lack of LPV resistance emergence in either group
- Lower Ctrough with LPV/r QD, not associated with reduced virologic response
- Limitation of the study: only 60% power to determine non inferiority of LPV/r QD
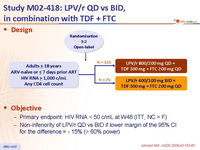
Design :

Objective :
- Primary endpoint: HIV RNA < 50 c/mL at W48 (ITT, NC = F)
- Non-inferiority of LPV/r QD vs BID if lower margin of the 95% CI for the difference = - 15% (> 60% power)
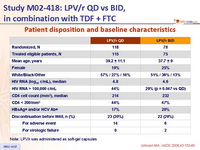
Patient disposition and baseline characteristics :

Response to treatment at week 48 :

Pharmacokinetics and resistance :
W4 steady-stade LPV PK
- BID group (N = 24) vs QD (N = 13)
- Cmax and AUC24 not significantly different
- Significantly lower Ctrough and Cmin for QD group (p < 0.003)
- Median Ctrough: 4.37 µg/mL for QD vs 6.64 µg/mL for BID
- Median IQ (Ctrough/IC50*) significantly lower for QD group (48.1) vs BID (86.5; p < 0.001)
*Protein-binding adjusted IC50 for wild-type HIV-1 = 0.07 mg/mL
Genotyping and phenotyping were performed in all specimens
with HIV RNA > 500 c/mL
from W12 through W48

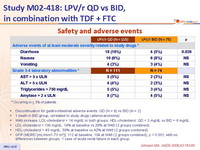
Safety and adverse events :

- Discontinuation for gastro-intestinal adverse events: QD (N = 9) vs BID (N = 2)
- 1 death in BID group, unrelated to study drugs (adenocarcinoma)
- W48 increase: LDL-cholesterol + 14 mg/dL in both groups; HDL-cholesterol: QD + 3 mg/dL vs BID + 6 mg/dL
- LDL-cholesterol > 130 mg/dL: 14% at baseline vs 26% at W48 (2 groups combined)
- HDL-cholesterol < 40 mg/dL: 58% at baseline vs 42% at W48 (2 groups combined)
- GFR (MDRD [mL/min/1.73 m2)]: 112 at baseline, 104 at W48 (2 groups combined), p < 0.001, with no differences between groups. 1 case of acute renal failure in each group.