Margolis DA. Lancet Infect Dis 2015; 15:1145-55
Phase 2 of new ARVs
» Cabotegravir (integrase inhibitor)
» CAB LA + RPV LA
CAB LA, RPV LA
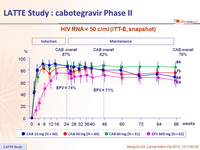
- Following 24 weeks induction therapy with 2 NRTIs and CAB, oral CAB + RPV maintained virologic suppression at a rate similar to EFV
+ 2 NRTIs through 96 weeks - CAB + RPV was well tolerated, with few drug-related AEs leading to discontinuation
- CAB 30 mg QD dose was selected for further oral development
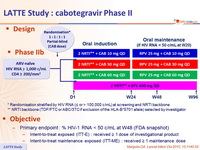
Design :
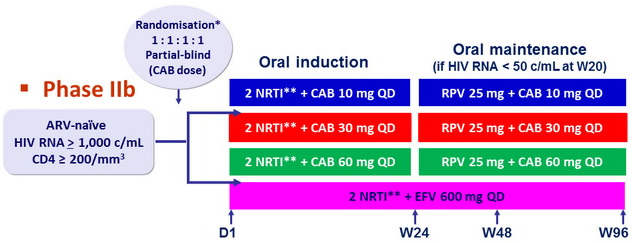
* Randomisation stratified by HIV RNA (≤ or > 100,000 c/ mL ) at screening and NRTI backbone
** NRTI backbone (TDF/FTC or ABC/3TC if exclusion of the HLA-B*5701 allele ) selected by investigator
Objective :
- Primary endpoint : % HIV-1 RNA < 50 c/mL at W48 (FDA snapshot)
- Intent-to-treat exposed (ITT-E) : received ≥ 1 dose of investigational product
- Intent-to-treat maintenance exposed (ITT-ME) : received ≥ 1 maintenance dose
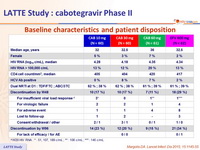
Baseline characteristics and patient disposition
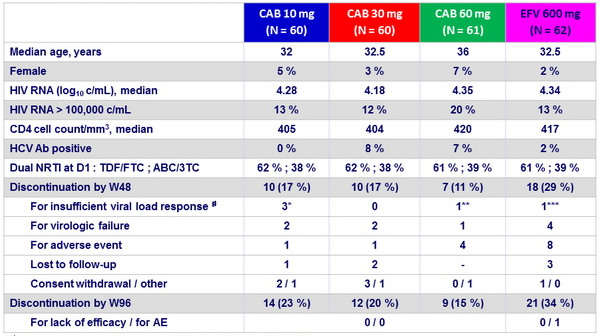
# W20 HIV RNA : * : 51, 107, 189 c/ mL ; ** : 108 c/ mL ; *** : 146 c/ mL
HIV RNA < 50 c/ml (ITT-E, snapshot )
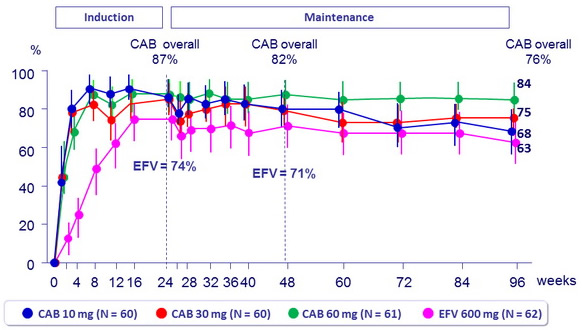
Week 96 outcome, ITT -ME, snaphsot
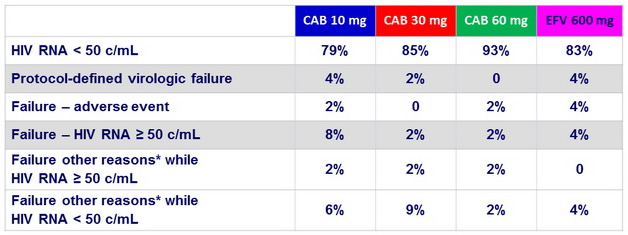
* Other reasons : missing data, protocol deviation, non-compliance, lost to follow-up, withdrawn consent, investigator discretion, ART change, ineligible for maintenance phase
Protocol-defined virologic failure (PDVF)
- Non-response: < 1 log 10 c/mL decrease of HIV RNA by Week 4, unless
< 400 c/mL ; or HIV RNA ≥ 200 c/mL on or after Week 16 - Rebound: HIV RNA ≥ 200 c/mL after confirmed < 200 c/mL ; or > 0.5 log 10 c/mL above nadir (the lowest prior HIV RNA ≥ 200 c/mL )
- Both non-response and rebound required consecutive confirmatory results
Protocol-defined virologic failures
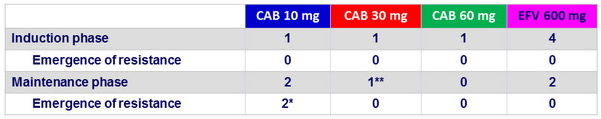
* CAB 10 mg : emergence of NNRTI (E138Q) and INI (Q148R) mutations at W48; CAB FC = 3, RPV FC = 2 ; CAB 10 mg : emergence of NNRTI mutations (K101K/E + E138E/A) but not to INI
Additional patient on CAB 10 mg : failure at W48 not confirmed, mutation to NNRTI (K101K/E + E138E/K), no INI mutation
** CAB 30 mg : PDVF at W36 with no emergence of NNRTI mutation ( integrase not amplified )
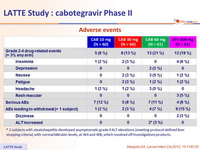
Adverse events
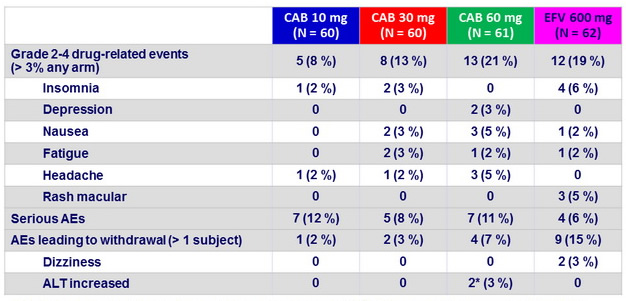
* 2 subjects with steatohepatitis developed asymptomatic grade 4 ALT elevations (meeting protocol-defined liver stopping criteria ) with normal bilirubin levels, at W4 and W8, which resolved off investigations products.
Laboratory abnormalities
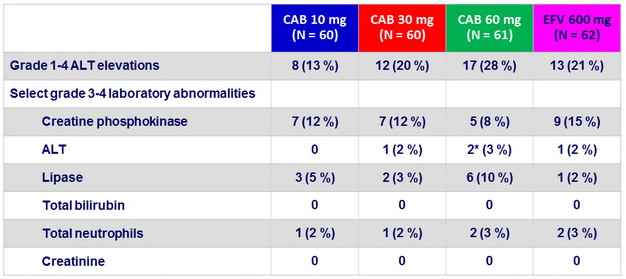 * 2 subjects with steatohepatitis developed asymptomatic grade 4 ALT elevations (meeting protocol-defined liver stopping criteria ) with normal bilirubin levels, at W8, which resolved off investigations products.
* 2 subjects with steatohepatitis developed asymptomatic grade 4 ALT elevations (meeting protocol-defined liver stopping criteria ) with normal bilirubin levels, at W8, which resolved off investigations products.