Bunupuradah T. Lancet HIV 2016;3:e343-50
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r reduced dose
» ATV 200/r + 2NRTI vs ATV 300/r + 2 NRTI
Switch studies in virologically suppressed patients
» Switch to PI/r reduced dose
» ATV 200/r + 2NRTI vs ATV 300/r + 2 NRTI
Drugs
ATV/r, 2 NRTI
ATV/r, 2 NRTI
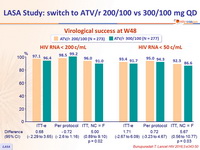
- ATV 200 mg and ritonavir 100 mg when combined with two NRTIs is non-inferior in terms of virological efficacy to ATV 300 mg and ritonavir 100 mg with two NRTIs in virologically suppressed Thai adults with HIV for use as second-line protease inhibitor-based ART
- When switches from randomised treatment were imputed as failures, the low-dose group was superior to the standard-dose group because the standard-dose group was associated with increased treatment discontinuation because of adverse events
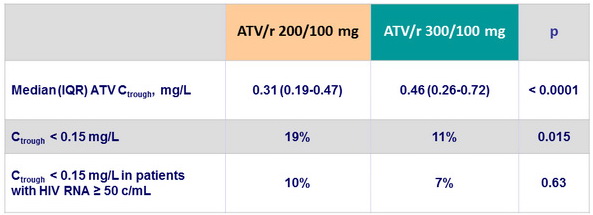
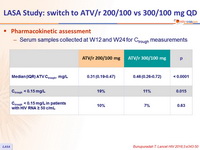
- More patients in the low-dose ATV group than in the standard-dose group had trough concentrations lower than the recommended therapeutic concentration of 0.15 mg/L
- Use of the low dose of ATV, with less toxicity than the standard dose, would benefit both patients and health-care systems (significant cost saving)
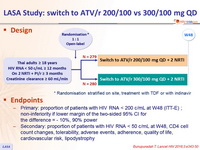
Design
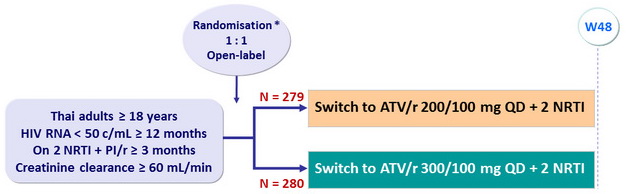
*
Randomisation stratified on site, treatment with TDF or with indinavir
Endpoints
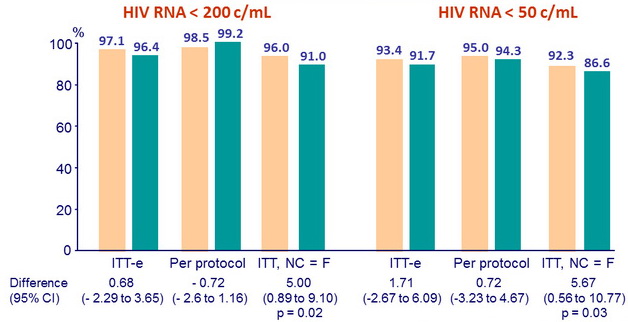
- Primary: proportion of patients with HIV RNA < 200 c/mL at W48 (ITT-E) ; non-inferiority if lower margin of the two-sided 95% CI for the difference = - 10%, 90% power
- Secondary: proportion of patients with HIV RNA < 50 c/mL at W48, CD4 cell count changes, tolerability, adverse events, adherence, quality of life, cardiovascular risk, lipodystrophy
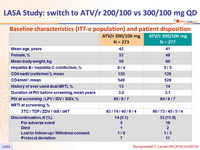
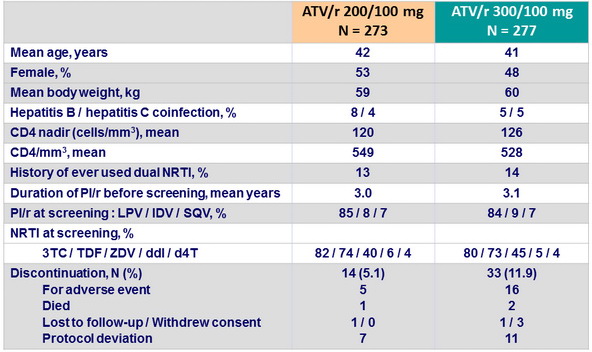
Baseline characteristics (ITT-e population) and patient disposition
Virological success at W48
◼ ATV/r 200/100 (n = 273) ◼ ATV/r 300/100 (n = 277)
Genotype Resistance testing
- Done in patients with protocol-defined virologic failure (confirmed HIV RNA ≥ 200 c/mL) and HIV RNA ≥ 1000 c/mL
- ATV/r 200/100, N = 7 ; emergence of resistance in 1: I50L, V82A, L90M + resistance to all NRTIs
- ATV/r 300/100, N = 1 ; no emergence of resistance
Study drug discontinuation
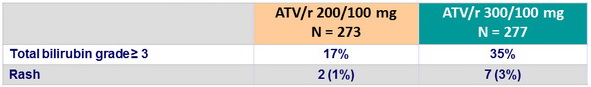
- ATV/r 200/100, N = 7 (3%): 1 death, 2 virologic failures, 2 rashes, 1 jaundice, 1 pregnancy
- ATV/r 300/100, N = 21 (8%): 1 death, 7 rashes, 6 jaundices, 1 pregnancy, 5 other reasons
Adverse events
- Similar proportion in the 2 treatment groups
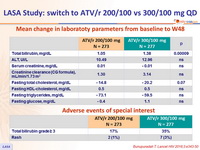
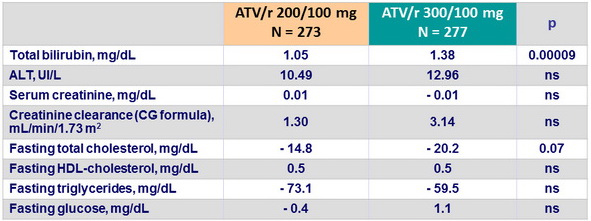
Mean change in laboratoty parameters from baseline to W48
Adverse events of special interest
Pharmacokinetic assessment
- Serum samples collected at W12 and W24 for Ctrough measurements