Nunes EP. HIV Clin Trials. 2009 Nov-Dec;10(6):368-74
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» LPV/r mono
Switch studies in virologically suppressed patients
» Switch to PI/r monotherapy
» LPV/r mono
Drugs
PI/r mono, LPV/r
PI/r mono, LPV/r
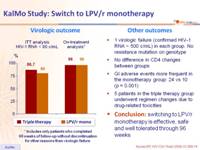
- Switching to LPV/r monotherapy is effective, safe and well tolerated through 96 weeks
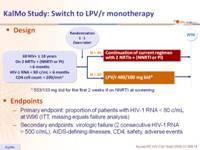
Design :
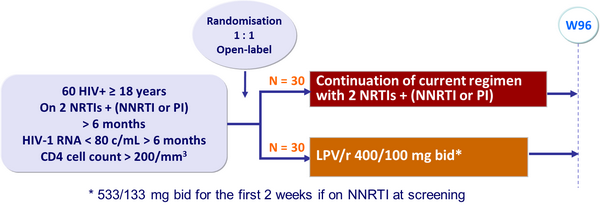
Endpoint :
- Primary endpoint: proportion of patients with HIV-1 RNA < 80 c/mL at W96 (ITT, missing equals failure analysis)
- Secondary endpoints: virologic failure (2 consecutive HIV-1 RNA > 500 c/mL), AIDS-defining illnesses, CD4, safety, adverse events
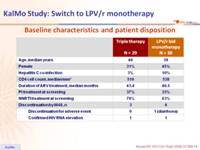
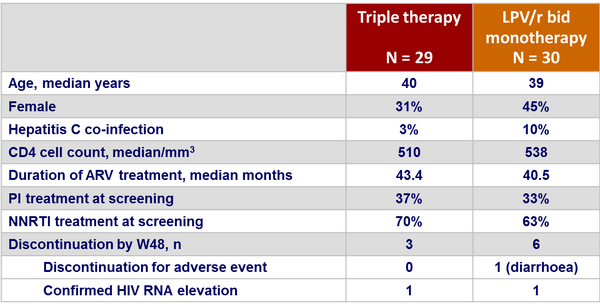
Baseline characteristics and patient disposition :
Virologic outcome :
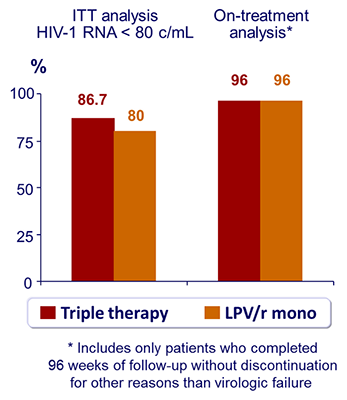
Virologic outcome :
- 1 virologic failure (confirmed HIV-1 RNA > 500 c/mL) in each group. No resistance mutation on genotype
- No difference in CD4 changes between groups
- GI adverse events more frequent in the monotherapy group: 24 vs 10 (p = 0.001)
- 5 patients in the triple therapy group underwent regimen changes due to drug-related toxicities