Smith KY. AIDS. 2009 Jul 31;23(12):1547-56
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» NRTI combinations
» ABC/3TC + LPV/r vs FTC/TDF + LPV/r
Head-to-head comparative trials for first line ART since 2006
» NRTI combinations
» ABC/3TC + LPV/r vs FTC/TDF + LPV/r
Drugs
LPV/r, FTC/TDF, ABC/3TC, TDF, ABC, FTC, 3TC
LPV/r, FTC/TDF, ABC/3TC, TDF, ABC, FTC, 3TC
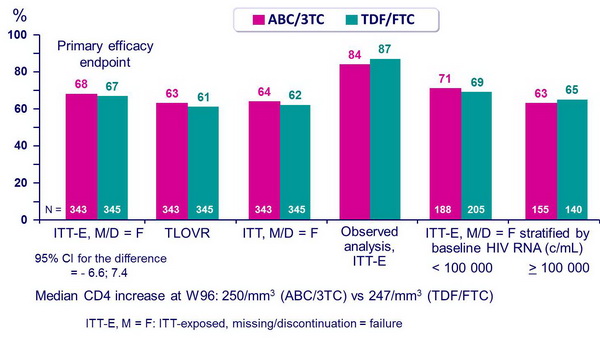
- As initial antiretroviral regimens, ABC/3TC and TDF/FTC, each in combination with LPV/r QD, have the same efficacy rate
- HIV RNA responses by baseline HIV RNA strata (< or > 100,000 c/mL) were similar between groups at W48 and W96
- Rate of virologic failure was similar in both groups (14%)
- CD4 response at W96 was similar in the 2 groups
- Both treatments were well tolerated
- More gastrointestinal intolerance with TDF/FTC
- More lipid abnormalities with ABC/3TC
- Of note, rate of discontinuation was high (34% at W96)
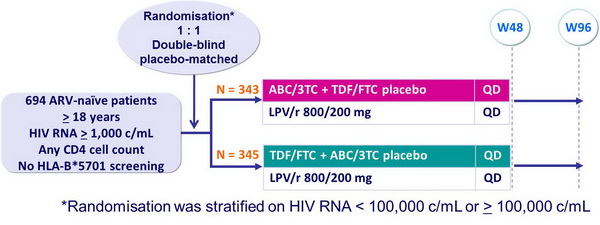
Design :
Objective :
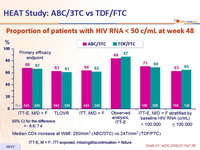
- Non inferiority of the 2 fixed dose NRTI combinations at W48: % HIV RNA < 50 c/mL, ITT-exposed, missing = failure [ITT-E, M = F] (lower margin of the 95% CI for the difference = - 12%, 90% power)
- Primary safety endpoint: incidence of adverse events at W96
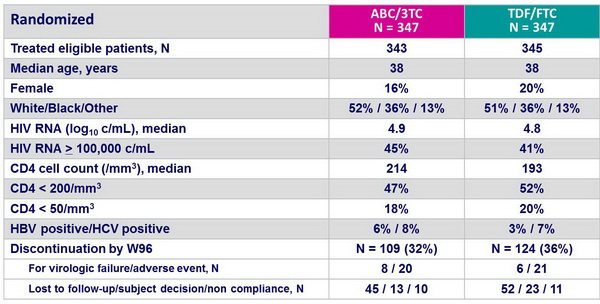
Patient disposition and baseline characteristics :
Note: change of NRTI (to NRTI other than ABC or TDF) allowed if intolerance; change of LPV/r QD to BID allowed if gastrointestinal intolerance, or to other PI if LPV/r-limiting intolerance. LPV/r was administered as soft-gel capsules (6/d) to week 48 then as tablets (4/d)
Proportion of patients with HIV RNA < 50 c/mL at week 48 :
Safety and tolerability (median exposure = 96 weeks) :
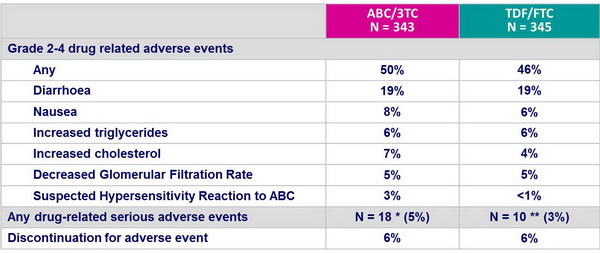
* Including suspected ABC HSR (N = 14), immune reconstitution syndrome (N = 2), hepatotoxicity (N = 1)
** Including suspected ABC HSR (N = 3), renal failure (N = 2), decreased creatinine renal clearance (N = 1)
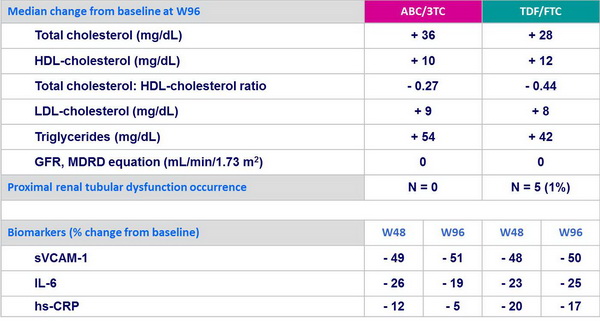
Change in laboratory parameters (lipids, renal, biomarkers) :