Sax PE. Lancet. 2017 Nov 4;390(10107):2073-2082; Stellbrink HJ. Lancet HIV 2019 ; 6:e364-72
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» INSTI vs INSTI
» BIC/FTC/TAF vs DTG + FTC/TAF
Head-to-head comparative trials for first line ART since 2006
» INSTI vs INSTI
» BIC/FTC/TAF vs DTG + FTC/TAF
Drugs
BIC/FTC/TAF, DTG, FTC/TAF
BIC/FTC/TAF, DTG, FTC/TAF
- Virologic suppression at W48 and W96 was high in both arms, with BIC/F/TAF being non inferior to DTG + F/TAF in treatment-naïve adults
- Sensitivity analyses confirmed BIC/F/TAF was non inferior to DTG + F/TAF
- No patient discontinued either treatment arm due to lack of efficacy
- No treatment-emergent resistance to any study medication was observed in either arm
- BIC/F/TAF was safe and well tolerated
- Less decrease in eGFR CG was observed with BIC/F/TAF vs DTG + F/TAF at W48
- There were no discontinuations due to renal adverse events and no cases of renal tubulopathy, including Fanconi syndrome, in either treatment group
- Changes from baseline in lipid parameters were equivalent
Design
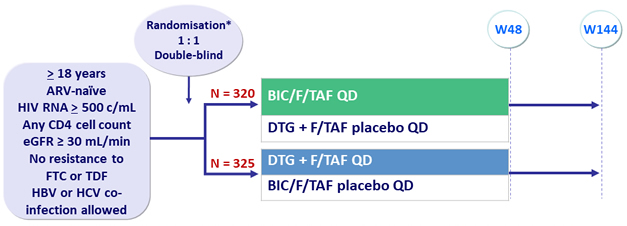
* Randomisation was stratified by HIV RNA (≤ 100 000 c/mL, 100 000-4000 000 c/mL or > 100 000 c/mL), CD4 (< 50/mm3, 50-199/mm 3 or ≥ 200/mm3) at screening and geographic region (USA vs non-USA)
BIC/F/TAF: 50/200/25 mg, as STR
Objective
- Non inferiority of BIC/F/TAF at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (lower margin of the 2-sided 95.002% CI for the difference= -12%, 95% power)
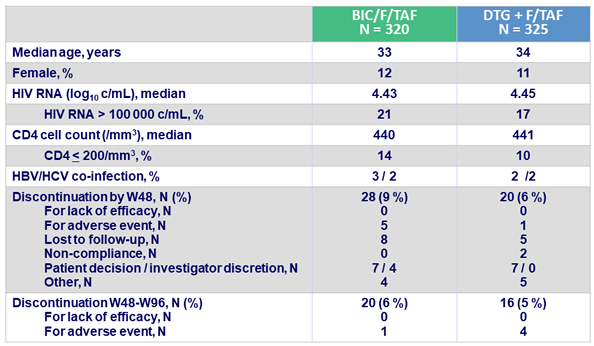
Baseline characteristics and patient disposition
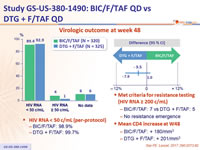
Virologic outcome at week 48
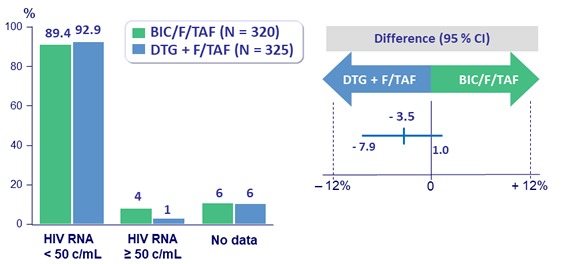
- HIV RNA < 50 c/ mL (per- protocol )
- BIC/F/TAF: 98.9%
- DTG + F/TAF: 99.7%
- Met criteria for resistance testing (HIV RNA ≥ 200 c/mL)
- BIC/F/TAF: 7 vs DTG + F/TAF: 5
- No resistance emergence
- Mean CD4 increase at W48
- BIC/F/TAF: + 180/mm3
- DTG + F/TAF: + 201/mm3
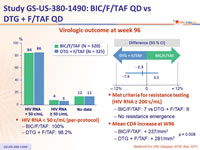
Virologic outcome at week 96
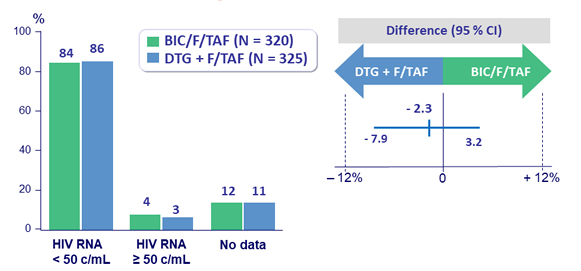
- HIV RNA < 50 c/ mL (per- protocol )
- ‒ BIC/F/TAF: 100%
- DTG + F/TAF: 98.2%
- Met criteria for resistance testing (HIV RNA ≥ 200 c/mL)
- BIC/F/TAF: 7 vs DTG + F/TAF: 6
- No resistance emergence
- Mean CD4 increase at W48
- BIC/F/TAF: + 237/mm3
- DTG + F/TAF: + 281/mm3
Adverse events at W48
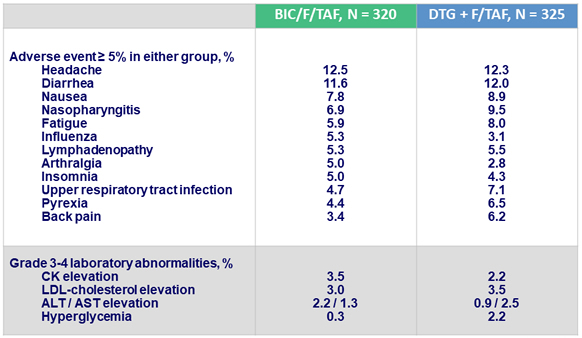
Drug-related adverse events at W96, %
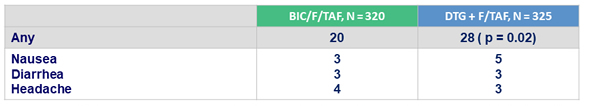
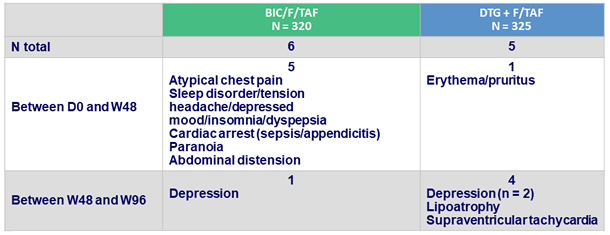
Adverse events leading to study drug discontinuation, D0-W96, N
Median change from baseline in eGFR and lipids
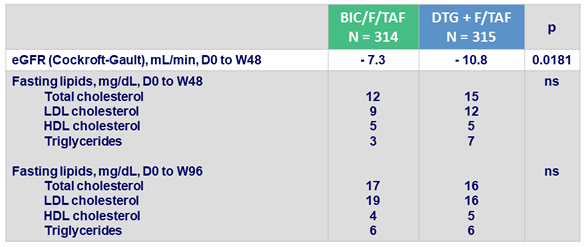
- No discontinuations due to renal adverse events and no proximal tubulopathy in either arm
Steady-state pharmacokinetic parameters of BIC/F/TAF (N = 17)
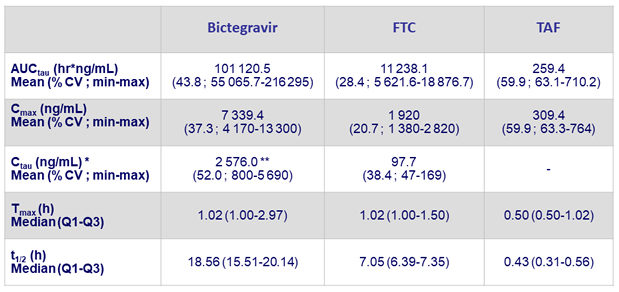
* N = 15
** BIC mean Ctau about 16 times higher than the protein adjusted effective concentration (162 ng/mL) against wild type HIV-1 virus