Kityo C CROI 2018, Abs. 500
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to BIC/FTC/TAF
» BIC/FTC/TAF vs E/C/F/TDF or E/C/F/TAF or ATV/r + FTC/TDF
Switch studies in virologically suppressed patients
» Switch to BIC/FTC/TAF
» BIC/FTC/TAF vs E/C/F/TDF or E/C/F/TAF or ATV/r + FTC/TDF
Drugs
BIC/FTC/TAF, E/C/F/TAF, E/C/F/TDF, ATV/r, FTC/TAF, FTC/TDF, TAF, TDF, FTC
BIC/FTC/TAF, E/C/F/TAF, E/C/F/TDF, ATV/r, FTC/TAF, FTC/TDF, TAF, TDF, FTC
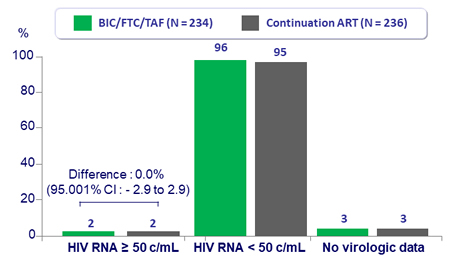
- Switching to BIC/FTC/TAF was non inferior to continuing ATV-
and EVG-based regimens at Week 48, in women- 1.7% of participants in both groups had HIV-1 RNA ≥ 50 c/mL
- 96% of women treated with BIC/FTC/TAF maintained
HIV-1 RNA < 50 c/mL vs 95% with continuation of ART
- No treatment-emergent resistance was observed in women receiving BIC/FTC/TAF
- BIC/FTC/TAF was well tolerated, and no adverse event led to discontinuation
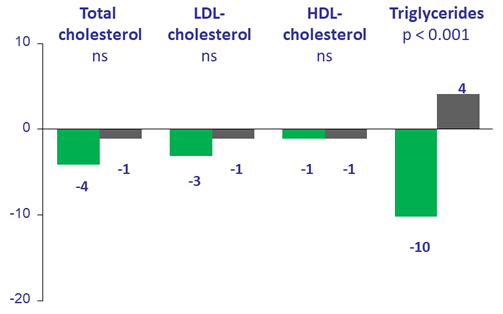
- Changes from baseline in lipid parameters and renal markers were comparable between treatment arms
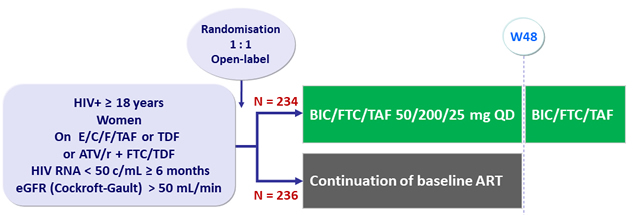
Design
Endpoints
- Primary: proportion of patients with HIV RNA ≥ 50 c/mL at W48 (ITT, snapshot) ; non-inferiority if upper margin of a two-sided 95.001% CI for the difference = 4%
- Secondary: proportion of patients with HIV RNA < 50 c/mL at W48 (ITT, snapshot)
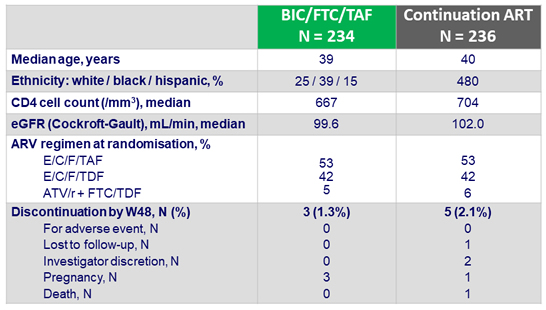
Baseline characteristics and patient disposition
Virologic outcome at W48
- Emergence of resistance in BIC/FTC/TAF: 0/1 patient analysed for resistance
- Emergence of resistance in Continuation ART:1/2 patients analysed for resistance (M184I/V)
Adverse events between D0 and W48, %
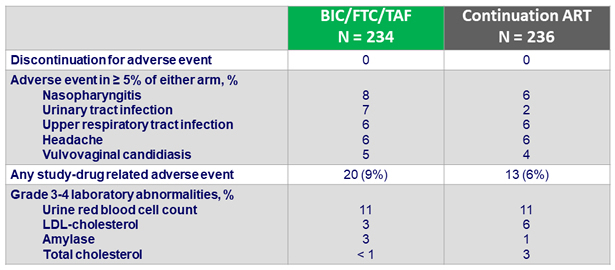
* Headache (N = 2), vomiting (N = 1), cerebrovascular accident (N = 1), abnormal dreams (N = 1),
suicidal ideation (N = 1) ; ** headache (N = 1), pruritus (N = 1)
Median change in eGFRCG at W48
- - 1.8 mL/min BIC/FTC/TAF vs - 2.7 mL/min Continuation ART (p = 0.70)
Median percent change in quantitative proteinuria at W48
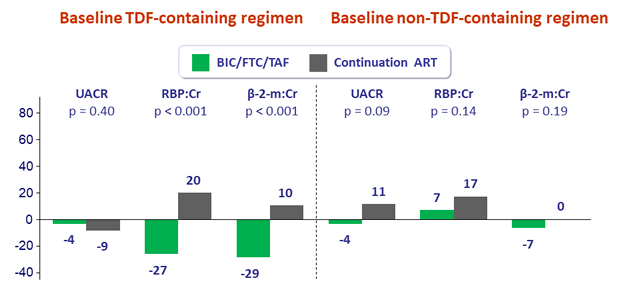
UACR: urine albumin:creatinine ratio ; RBP: retinol-binding protein ; β-2- m: beta-2 microglobulin
Median % change in fasting lipids at W48