Huhn GD. JAIDS 2017; 74:193.200
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to E/C/F/TAF + DRV/r
Switch studies in virologically suppressed patients
» Switch to E/C/F/TAF + DRV/r
Drugs
E/C/F/TAF, EVG/c, DRV/r, FTC/TAF, TAF, FTC
E/C/F/TAF, EVG/c, DRV/r, FTC/TAF, TAF, FTC
- Simplifying therapy from ~5 pills/day to once-daily, 2-pill E/C/F/TAF + DRV
- Provided efficacious plasma exposures of EVG, DRV, and TAF
- Maintained virologic suppression through Week 24
- Was superior to staying on baseline regimen at Week 48 at both < 50 and < 20 c/mL
- Switch to TAF improved proximal tubular proteinuria without change in eGFR
- E/C/F/TAF + DRV was safe, well tolerated, and associated with greater treatment satisfaction
- For treatment-experienced individuals with ≥ 2 class resistance on complex, high-pill burden regimens, switching to E/C/F/TAF
+ DRV provides a simple, once-daily, two-pill option with superior efficacy and comparable tolerability
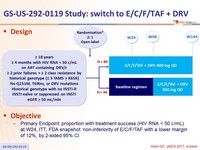
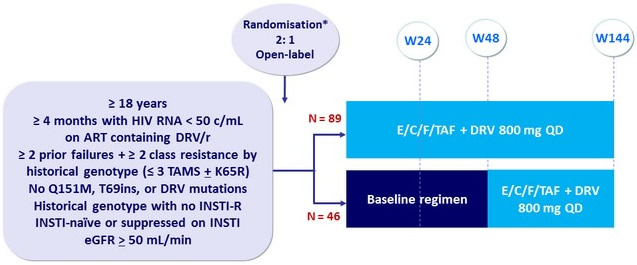
Design
Objective
- Primary Endpoint: proportion with treatment success (HIV RNA < 50 c/mL) at W24, ITT, FDA snapshot: non-inferiority of E/C/F/TAF with a lower margin of 12%, by 2-sided 95% CI
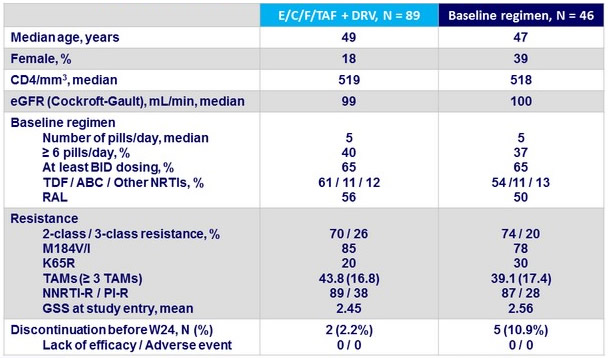
Baseline characteristics and disposition at W48
Pharmacokinetic substudy Results (N = 15)
- Once-daily dosing of E/C/F/TAF (150/150/200/10 mg) + DRV 800 mg
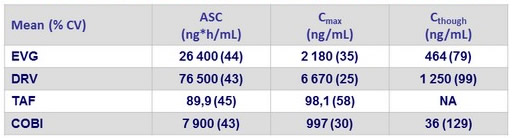
- EVG Ctrough >10-fold above IC 95 (45 ng /mL)
- DRV Ctrough>22-fold above EC 50 (55 ng /mL)
- TAF exposures in efficacious range demonstrated in pivotal Phase 3 studies
- COBI exposure associated with robust boosting
- TFV exposure (mean [%CV] AUC 367 [33] ng *h/mL) well below levels observed following TDF-containing regimens
Efficacy and Safety Results
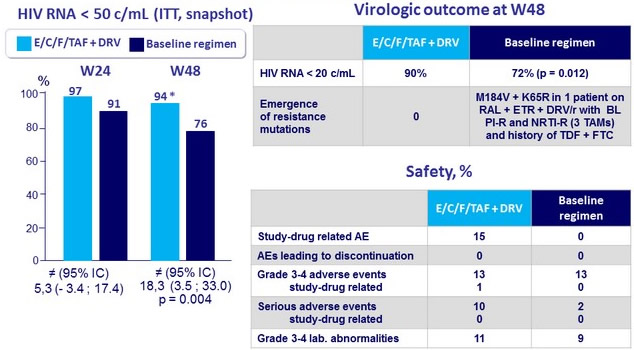
* 91% if prior DRV/r dose 800 QD vs 100% if prior DRV/r dose 600/100 BID