Walmsley S. J Acquir Immune Defic Syndr. 2009 Apr 1;50(4):367-74
Type of ARV Trial
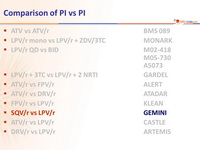
Head-to-head comparative trials for first line ART since 2006
» PI vs PI
» SQV/r + FTC/TDF vs LPV/r + FTC/TDF
Head-to-head comparative trials for first line ART since 2006
» PI vs PI
» SQV/r + FTC/TDF vs LPV/r + FTC/TDF
Drugs
LPV/r, SQV/r, FTC/TDF, TDF, FTC
LPV/r, SQV/r, FTC/TDF, TDF, FTC
- SQV/r BID was non inferior to LPV/r BID, in combination with TDF/FTC fdc
- Virologic and immunologic responses were similar in both arms
- Tolerability was similar in both arms
- Gastrointestinal adverse events were more frequent with LPV/r
- Lipid changes were not different between SQV/r and LPV/r except for triglycerides elevation, which was higher with LPV/r
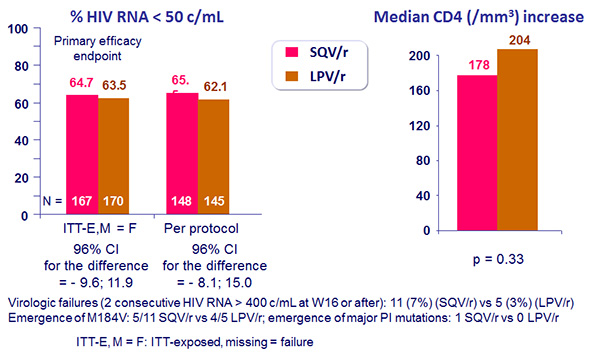
- Rate of virologic failure was low in both groups
- 1 patient in the SQV/r group developed new major protease resistance mutations at virologic failure
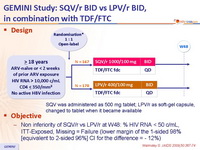
Design :
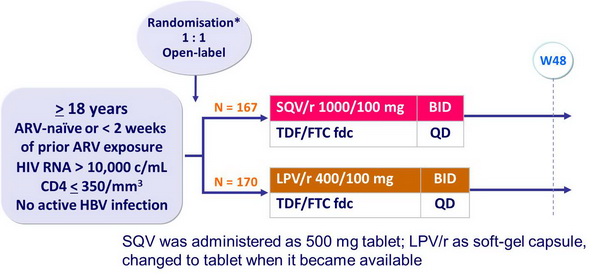
Objective :
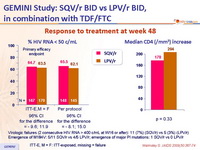
- Non inferiority of SQV/r vs LPV/r at W48: % HIV RNA < 50 c/mL, ITT-Exposed, Missing = Failure (lower margin of the 1-sided 98% [equivalent to 2-sided 96%] CI for the difference = - 12%)
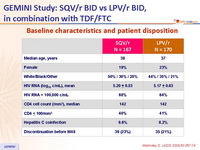
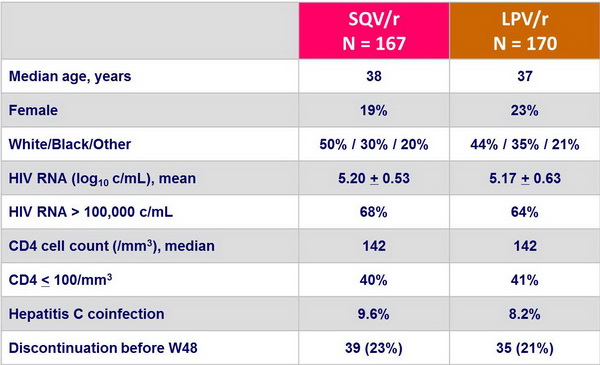
Baseline characteristics and patient disposition :
Response to treatment at week 48 :
Safety and tolerability: SQV/r vs LPV/r :
- Low frequency of premature discontinuations for adverse events: 3% vs 7%
- Most frequent reported adverse events of any grade were gastrointestinal disorders: 17% vs 27%
- No discontinuation because of renal-related adverse events; 2 patients, in the LPV/r arm, had elevated plasma creatinine levels > 2 mg/dL attributed to TDF/FTC
- Median changes at W48 in total, LDL- and HDL-cholesterol were not significantly different between treatment groups; elevation of triglycerides was significantly higher in the LPV/r arm