Clotet B. Lancet. 2014 Jun 28;383(9936):2222-31
Type of ARV Trial
Head-to-head comparative trials for first line ART since 2006
» INSTI vs PI
» DTG + 2NRTI vs DRV/R + 2 NRTI
Head-to-head comparative trials for first line ART since 2006
» INSTI vs PI
» DTG + 2NRTI vs DRV/R + 2 NRTI
Drugs
DTG, DRV/r, 2 NRTI
DTG, DRV/r, 2 NRTI
Conclusion at week 48
- DTG 50 mg QD achieved higher virologic success at week 48, than DRV/r QD, when combined with either TDF/FTC or ABC/3TC
- In patients with high baseline viral load, the response rate was higher for DTG
- No resistance mutations were detected through 48 weeks in the 2 groups
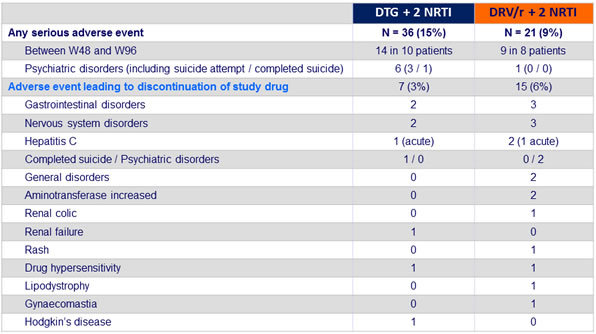
- Adverse events leading to discontinuation occurred less frequently in the DTG group
- No specific trends in adverse events
- With the exception of 2 patients reporting suicide attempt and overdose on DTG
- No discontinuation due to renal events
- Mean increases in creatinine with accompanying decreases in estimated glomerular filtration rate occurred by week 4, and stabilized up to week 48
- Once-daily DTG in combination with fixed-dose NRTIs represents an effective treatment option for HIV-1-infected, treatment-naive patients
Conclusion at week 96
- Durable suppression of viral replication with DTG 50 mg + 2 NRTIs with no new cases of virological failure after 48 weeks
Design :
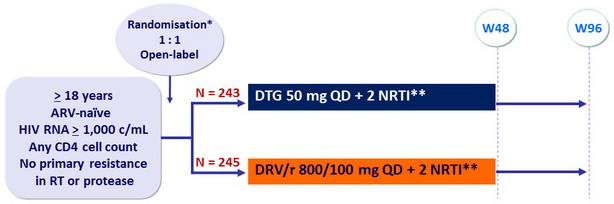
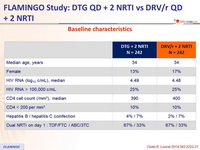
*Randomisation (DTG vs DRV/r) was stratified by HIV RNA ( < or > 100,000 c/mL) at screening and NRTI backbone
**NRTI backbone (TDF/FTC or ABC/3TC if exclusion of the HLA-B*5701 allele) was selected by investigator
Objective :
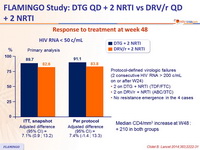
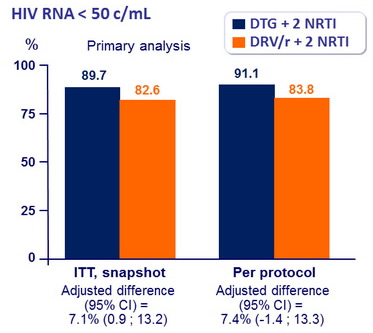
- Non inferiority of DTG at W48: % HIV RNA < 50 c/mL by intention to treat, snapshot analysis (1-sided significance level of 2.5%, lower margin of the 95% CI for the difference = -12%, 90% power)
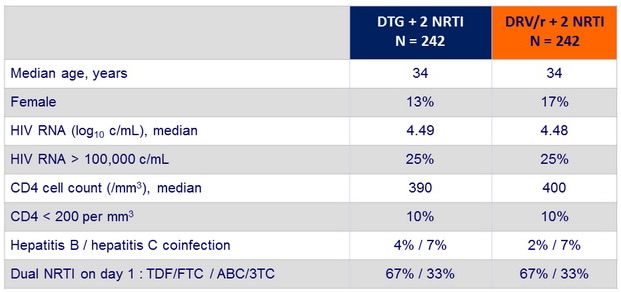
Baseline characteristics :
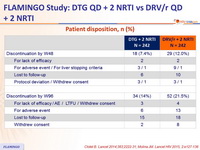
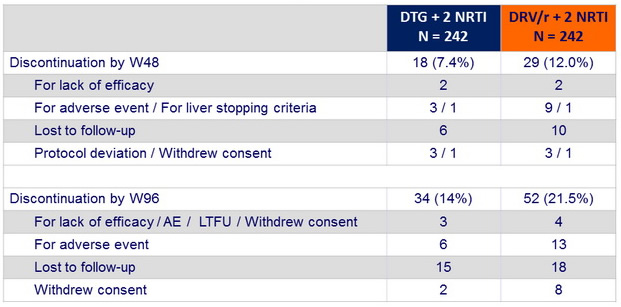
Patient disposition, n (%)
Response to treatment at week 48 :
Protocol-defined virologic failures
(2 consecutive HIV RNA > 200 c/mL
on or after W24)
- 2 on DTG + NRTI (TDF/FTC)
- 2 on DRV/r + NRTI (ABC/3TC)
- No resistance emergence in the 4 cases
Median CD4/mm 3 increase at W48 :
+ 210 in both groups
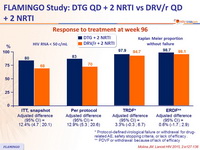
Response to treatment at week 96
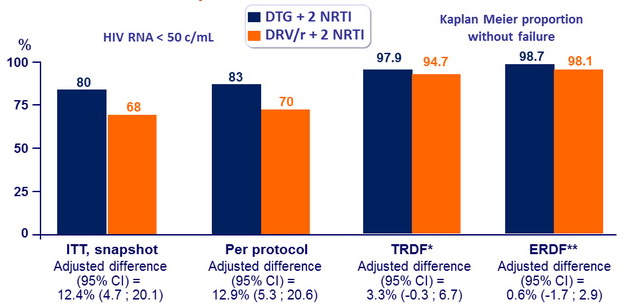
* Protocol- defined virological failure or withdrawal for drug-related AE, safety stopping criteria, or lack of efficacy
** PDVF or withdrawal because of lack of efficacy
HIV-1 RNA < 50 c/mL at week 48 by stratification factors (HIV-1 RNA and background NRTI)
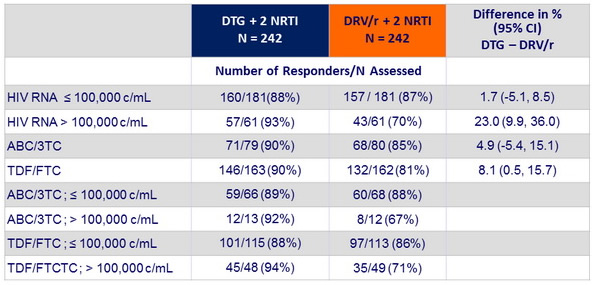
HIV-1 RNA < 50 c/mL at week 96 by stratification factors (HIV-1 RNA and background NRTI)
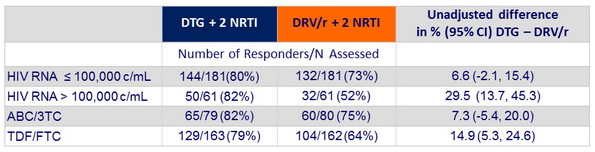
- Median CD4/mm3 increase at W96 :
- + 260 (IQR 185 – 400) in the DTG group
- + 250 (IQR 130-400) in the DRV/ r group
- Protocol-defined virologic failures (2 consecutive HIV RNA > 200 c/mL on or after W24)
- 2 on DTG + NRTI (TDF/FTC) ; 4 on DRV/ r + NRTI
- No resistance emergence in the 6 cases
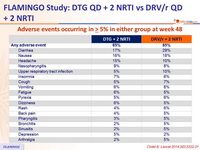
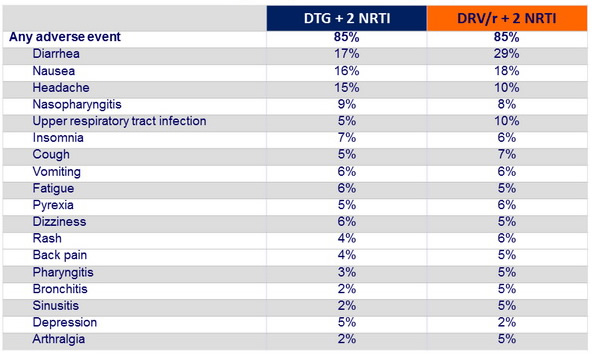
Adverse events occurring in ≥ 5% in either group at week 48 :
Safety at week 48 :
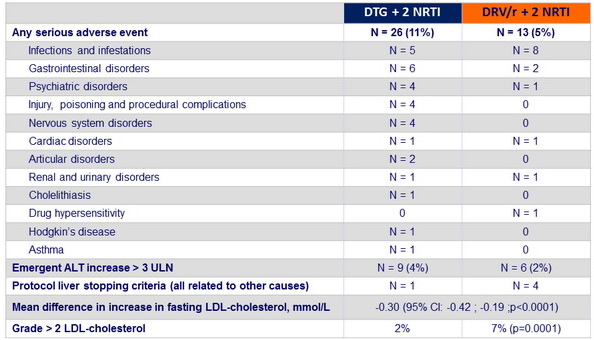
Safety at week 96 :