Orkin C. Lancet HIV. 2018 Jan;5(1):e23-e34.
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to D/C/F/TAF
» D/C/F/TAF vs IP/r + FTC/TDF
Switch studies in virologically suppressed patients
» Switch to D/C/F/TAF
» D/C/F/TAF vs IP/r + FTC/TDF
Drugs
D/C/F/TAF, DRV/c, FTC/TAF, FTC/TDF, TAF, TDF, FTC
D/C/F/TAF, DRV/c, FTC/TAF, FTC/TDF, TAF, TDF, FTC
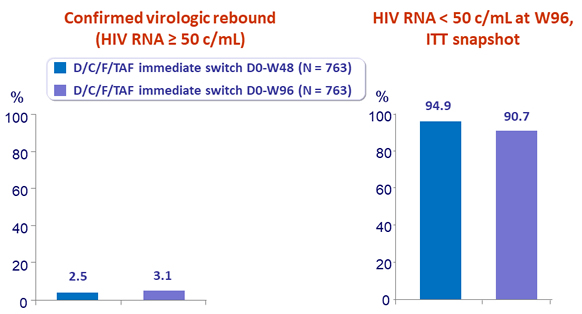
- Through Week 96, switching from boosted PI + FTC/TDF to D/C/F/TAF resulted in:
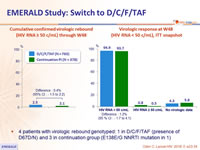
- Low virologic rebound rate cumulative (2.5% at W48, 3.1 % at W96)
- High virologic suppression rate (94.9% at W48, 91% at W96)
- Virologic non-response rate of 1%
- No resistance to any study drug
- Few serious adverse events and discontinuations due to adverse events (2%)
- D/C/F/TAF bone, renal and lipid safety were consistent with known profiles of TAF and cobicistat
Design

*
Randomisation stratified by boosted PI
Primary endpoint
- Proportion of patients with virologic rebound at W48 ; non-inferiority if lower margin of a two-sided 95% CI for the adjusted difference = - 4%, 89% power
- Virologic rebound: confirmed HIV RNA ≥ 50 c/mL (or single HIV RNA > 50 c/mL at W48), or premature discontinuation, irrespective of reason, with last HIV RNA ≥ 50 c/mL through W48
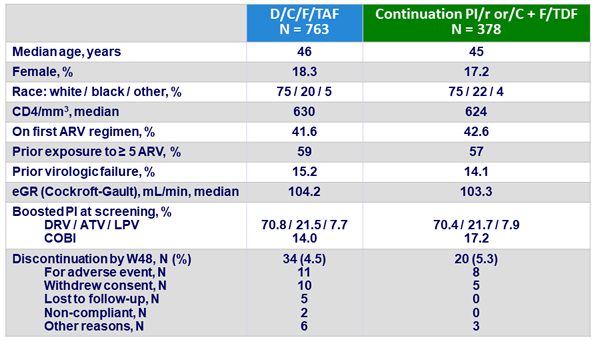
Baseline characteristics and patient disposition
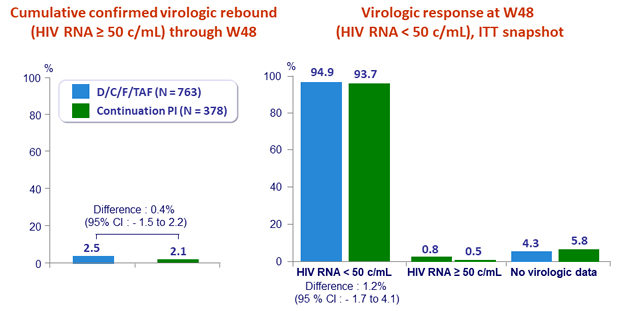
- 4 patients with virologic rebound genotyped: 1 in D/C/F/TAF (presence of D67D/N) and 3 in continuation group (E138E/G NNRTI mutation in 1)
Virologic rebound rate through W48 according to previous antiretroviral failure
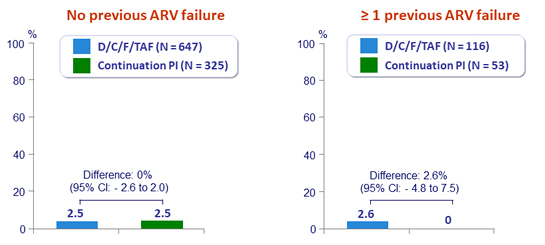
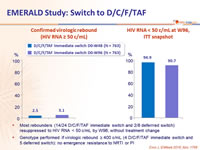
- Most rebounders (14/24 D/C/F/TAF immediate switch and 2/8 deferrred switch) resuppressed to HIV RNA < 50 c/mL by W96, without treatment change
- Genotype performed if virologic rebound ≥ 400 c/mL (4 D/C/F/TAF immediate switch and 5 deferred switch): no emergence resistance to NRTI or PI
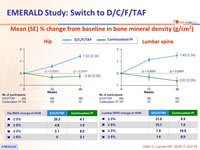
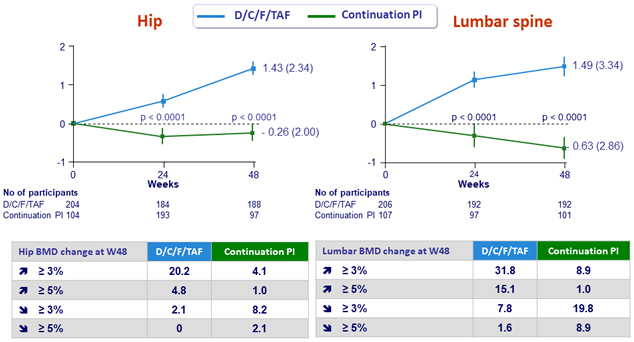
Mean (SE) % change from baseline in bone mineral density (g/cm²)
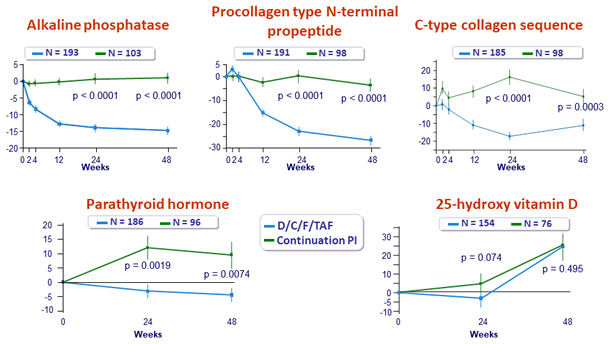
Mean (SE) % change from baseline in bone biomarkers
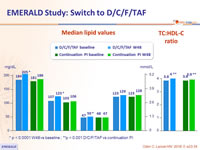
Median lipid values
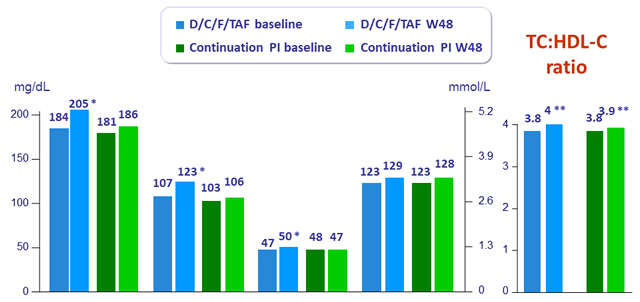
* p < 0.0001 W48 vs baseline ; **p < 0.001 D/C/F/TAF vs continuation PI
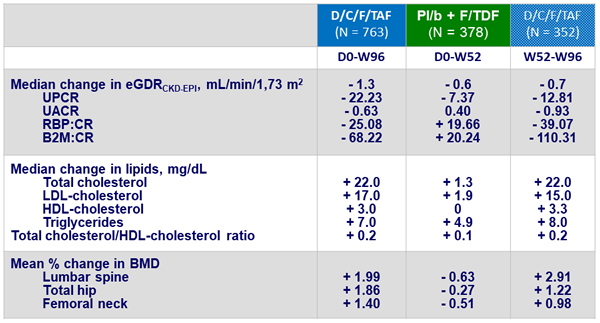
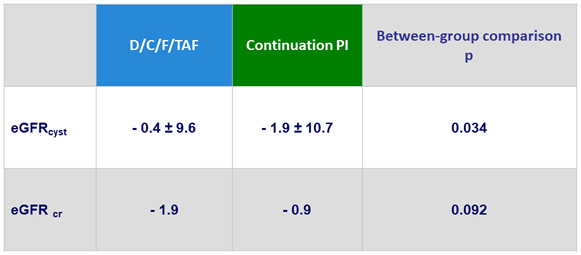
Mean Changes in eGFR (mL/min/1.73 m²) at W48
Adverse events between D0 and W48, %
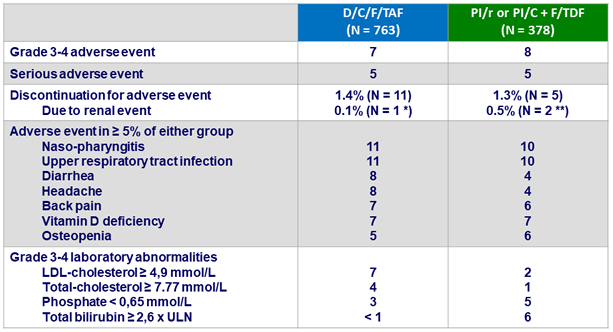
* Worsening of pre-existing renal insufficiency ; ** Toxic nephropathy, N = 1 ; tubulopathy , N = 1
Adverse events, D0-W96 (%)
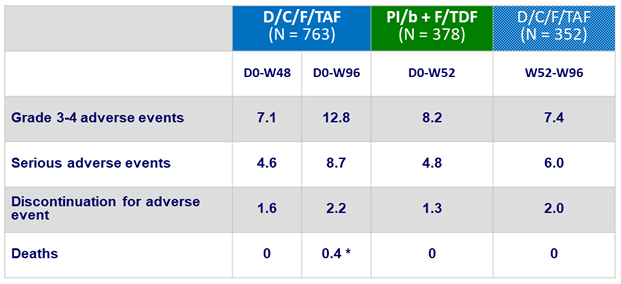
* 1 metastatic pancreas cancer and 2 myocardial infarction
Changes in renal, lipid and bone parameters at W96 (%)