Molina JM. Lancet. 2011 Jul 16;378(9787):238-46 ; Cohen CJ. Lancet. 2011 Jul 16;378(9787):229-37 & AIDS. 2013 Mar 27;27(6):939-50 ; Rimsky L. J Acquir Immune Defic Syndr. 2012 Jan 1;59(1):39-46 & Antivir Ther. 2013;18(8):967-77
Head-to-head comparative trials for first line ART since 2006
» NNRTI vs NNRTI
» EFV + FTC/TDF or 2 NRTI vs RPV + FTC/TDF or 2 NRTI
RPV, EFV 600, 2 NRTI, FTC/TDF, TDF, FTC
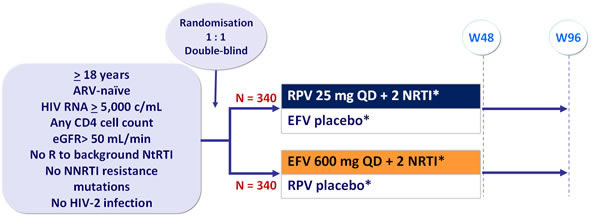
ECHO Study: RPV + TDF/FTC QD vs EFV + TDF/FTC QD
Design :
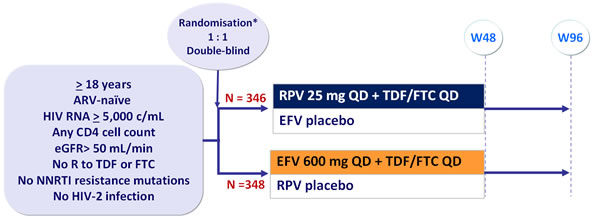
* Randomisation was stratified by HIV RNA (< or > 100,000 c/mL, < 500,000 or > 500,000 c/mL ) at screening
- RPV taken with meal, EFV taken on an empty stomach in the evening
Objective :
- Non inferiority of RPV vs EFV at W48: % HIV RNA < 50 c/mL by intention to treat, TLOVR analysis (lower margin of the 2-sided 95% CI for the difference = 12%, 95% power)
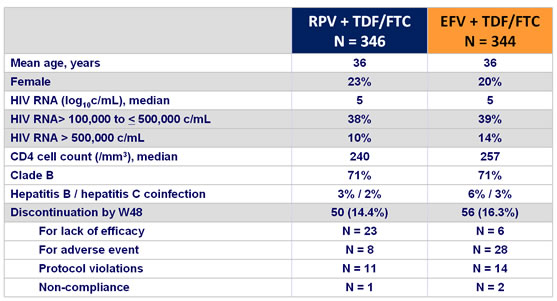
Baseline characteristics and patient disposition :
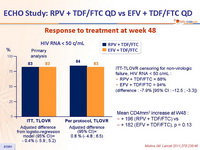
Response to treatment at week 48 :
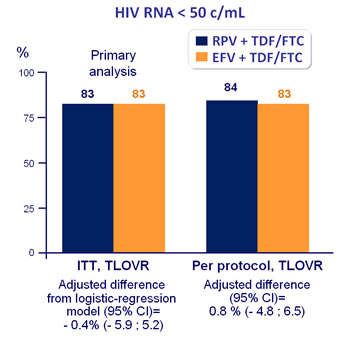 ITT-TLOVR censoring for non-virologic failure, HIV RNA < 50 c/mL :
ITT-TLOVR censoring for non-virologic failure, HIV RNA < 50 c/mL :
-
RPV + TDF/FTC = 86%
-
EFV + TDF/FTC = 94%
(difference : -7.9% [95% CI : -12.5 ; -3.3])
Mean CD4/mm3 increase at W48 :
+ 196 (RPV + TDF/FTC) vs
+ 182 (EFV + TDF/FTC), p = 0.13
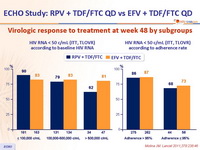
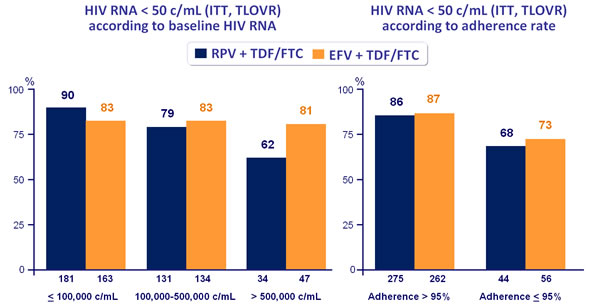
Virologic response to treatment at week 48 by subgroups :
Virologic failure definition :
- Never suppressed : never achieved 2 consecutive HIV RNA < 50 c/mL and increase of HIV RNA ≥ 0.5 log10 c/mL above the nadir
- Rebounder : achieved 2 consecutive HIV RNA < 50 c/mL with 2 subsequent consecutive (or 1 if last available) HIV RNA ≥ 50 c/mL
Criteria for resistance testing :
- All virological failures
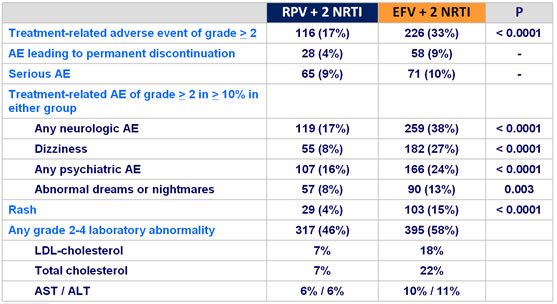
Treatment-emergent adverse events :
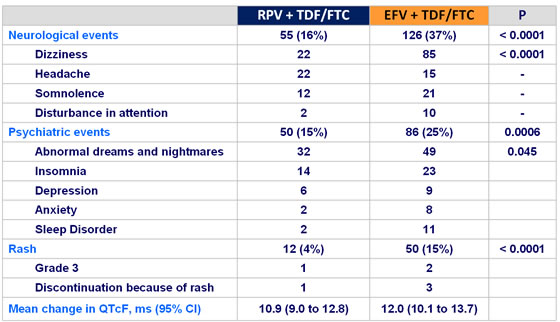
Adverse events of interest of any grade :
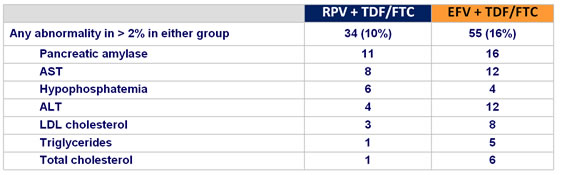
Grade 3-4 laboratory abnormalities :
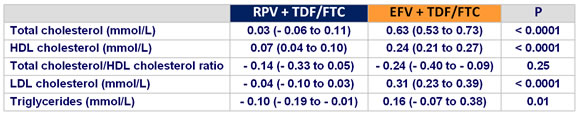
Mean (95% CI) change in fasting lipids from baseline to week 48 :
Summary of week 48 results :
- RPV QD is virologically non inferior to EFV, when given in combination with TDF/FTC
- Response rate was lower in the RPV group for patients with highest baseline viral loads
- Discontinuation because of virologic failure was higher for RPV, and discontinuation because of adverse events was higher for EFV
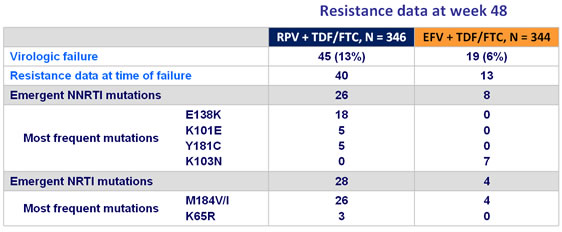
- Proportion of virological failures with
- ≥ 1 emergent NNRTI resistance-associated mutation : similar in both groups
- Most frequent mutations on RPV lead to NNRTI cross resistance
- At EFV failure, K103 N was the most frequent mutation, with conserved sensitivity to etravirine
- ≥ 1 emergent NRTI resistance mutation : higher in the RPV group
- ≥ 1 emergent NNRTI resistance-associated mutation : similar in both groups
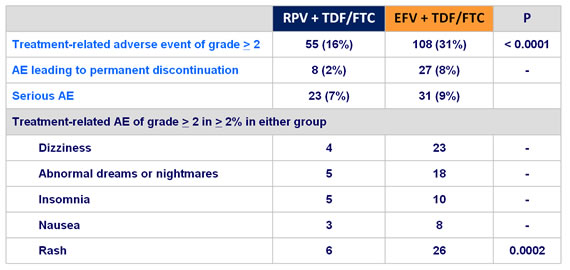
- More favorable overall safety profile of RPV than EFV : lower rate of
- grade 2-4 AE possibly related to treatment
- rash
- neurological and psychiatric adverse events
- increases in proatherogenic lipid parameters
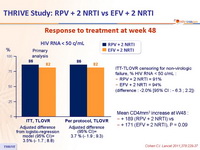
THRIVE Study: RPV + 2 NRTI vs EFV + 2 NRTI
Design :
Randomisation was stratified by HIV RNA (< or > 100,000 c/mL, < 500,000 or > 500,000 c/mL ) at screening
* Open-label NRTI combination selected by investigator : ZDV+3TC BID or ABC+3TC QD or TDF+FTC QD
Objective :
- Non inferiority of RPV vs EFV at W48: % HIV RNA < 50 c/mL by intention to treat, TLOVR analysis (lower margin of the 2-sided 95% CI for the difference = 12%, 95% power)
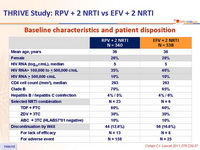
Baseline characteristics and patient disposition :
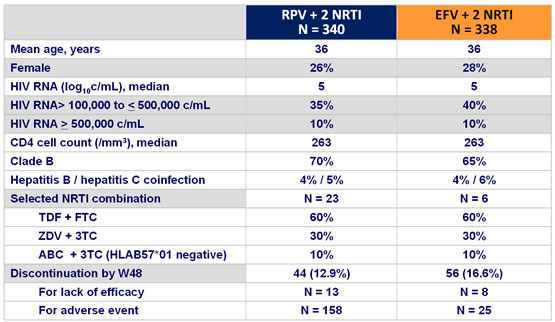
Response to treatment at week 48 :
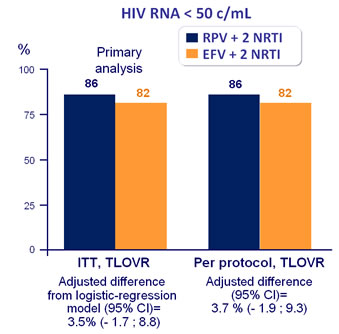
ITT-TLOVR censoring for non-virologic failure, % HIV RNA < 50 c/mL :
-
RPV + 2 NRTI = 91%
-
EFV + 2 NRTI = 94%
(difference : -2.0% [95% CI : - 6.3 ; 2.2])
Mean CD4/mm3 increase at W48 :
+ 189 (RPV + 2 NRTI) vs
+ 171 (EFV + 2 NRTI), P = 0.09
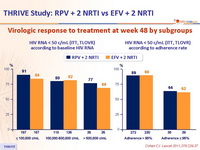
Virologic response to treatment at week 48 by subgroups :
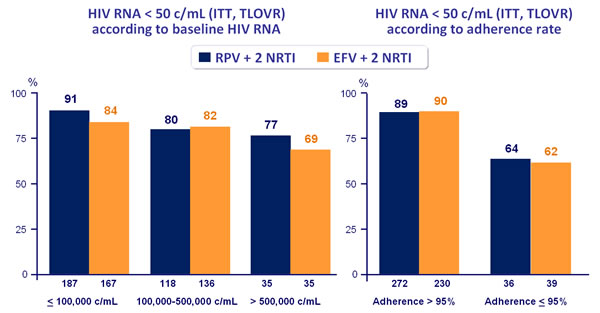
Virologic failure definition :
- Never suppressed : never achieved 2 consecutive HIV RNA < 50 c/mL and increase of HIV RNA ≥ 0.5 log10 c/mL above the nadir
- Rebounder : achieved 2 consecutive HIV RNA < 50 c/mL with 2 subsequent consecutive (or 1 if last available) HIV RNA ≥ 50 c/mL
Criteria for resistance testing :
- All virological failures
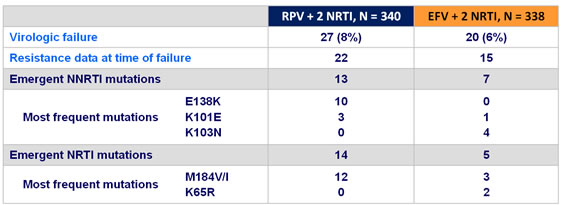
Treatment-emergent adverse events :
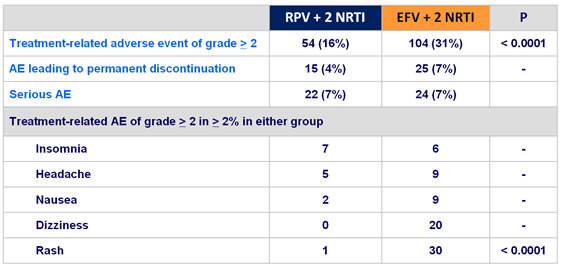
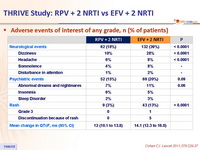
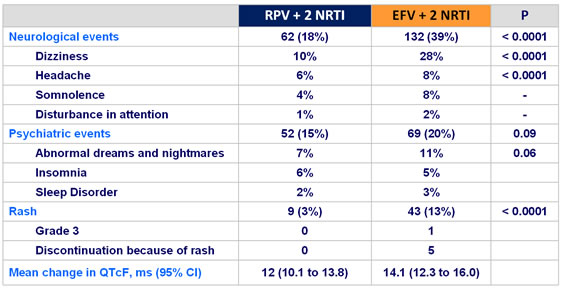
Adverse events of interest of any grade, n (% of patients) :
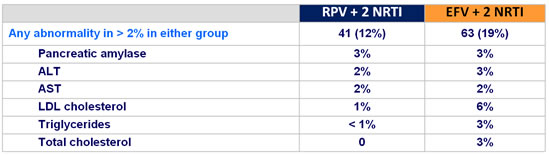
Grade 3-4 laboratory abnormalities :
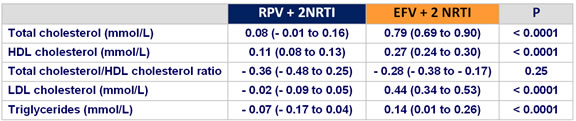
Mean (95% CI) change in fasting lipids from baseline to week 48 :
Summary of week 48 results :
- RPV QD is virologically non inferior to EFV, when given in combination with 2 NRTI
- Response rates seemed highest in the RPV group for patients with lowest baseline viral loads
- Background NRTI regimen had no significant effect on responses (limitation : no randomisation, no stratification by NRTI)
- Discontinuation because of adverse events or other reasons was lower for RPV
- Proportion of virological failures with
- ≥ 1 emergent NNRTI resistance-associated mutation : similar in both groups,
- ≥ 1 emergent NRTI resistance mutation : higher in the RPV group
- More favorable overall safety profile of RPV than EFV : lower rate of
- grade 2-4 AE possibly related to treatment
- rash
- dizziness
- increases in proatherogenic lipid parameters
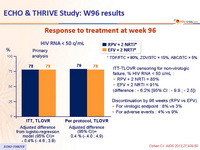
ECHO & THRIVE Study: W96 results
Response to treatment at week 96 :
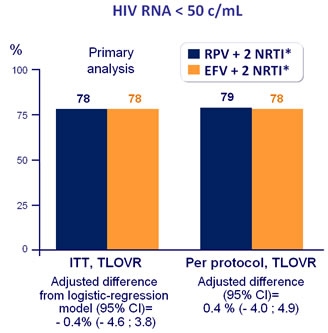
* TDF/FTC = 80%, ZDV/3TC = 15%, ABC/3TC = 5%
ITT-TLOVR censoring for non-virologic failure, % HIV RNA < 50 c/mL :
-
RPV + 2 NRTI = 85%
-
EFV + 2 NRTI = 91% (difference : - 6.2% [95% CI : - 9.9 ; - 2.5])
Discontinuation by 96 weeks (RPV vs EFV)
-
For virologic endpoint : 8% vs 3%
-
For adverse events : 4% vs 9%
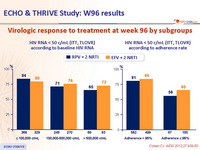
Virologic response to treatment at week 96 by subgroups :
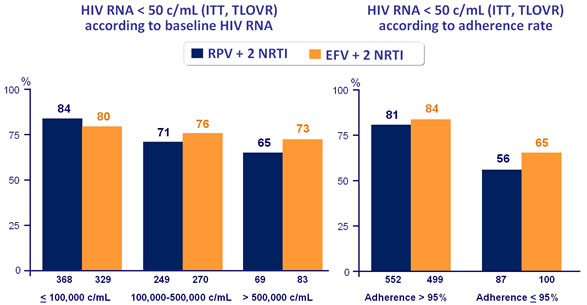
*Randomisation was stratified by clinical site and by HIV RNA (< or > 100,000 c/mL) at screening
- Non inferiority of EFV 400 mg at W48: % HIV RNA < 200 c/mL by modified intention to treat analysis (all randomised participants who received at least 1 dose of study drug and at least one follow-up visit), 2-sided significance level of 5%, lower margin of the 95% CI for the difference = -10%, 90% power
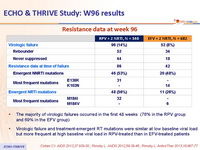
Resistance data at week 96 :
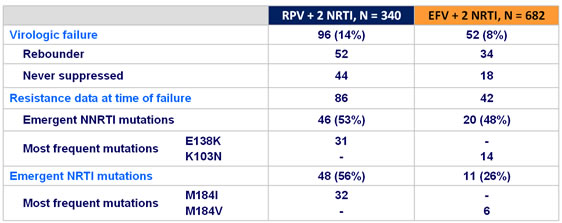
- The majority of virologic failures occurred in the first 48 weeks (76% in the RPV group and 69% in the EFV group)
- Virologic failure and treatment-emergent RT mutations were similar at low baseline viral load but more frequent at high baseline viral load in RPV-treated than in EFV-treated patients
Adverse events and treatment-emergent grade 2-4 laboratory abnormalities :