Molto J. J AntimicrobChemother 2015;70:1139-45
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r reduced dose
» DRV 600/r + 2 NRTI vs DRV 800/r + 2 NRTI
Switch studies in virologically suppressed patients
» Switch to PI/r reduced dose
» DRV 600/r + 2 NRTI vs DRV 800/r + 2 NRTI
Drugs
DRV/r, 2 NRTI
DRV/r, 2 NRTI
- The efficacy of a DRV daily dose of 600 mg seemed to be similar to the efficacy of the standard 800 mg dose, in combination with ritonavir 100 mg and 2 NRTI, in virologically suppressed HIV-infected patients switching from therapy with DRV/r 800/100 mg + 2 NRTI
- This strategy can potentially translate to substantial savings in the cost of care of HIV-infected patients
- Average reduction in annual cost per successfully treated DRV 600-arm patient of 7273 $US
- Limitation : trial not powered to detect differences in efficacy below 15%, which might be clinically relevant
Design
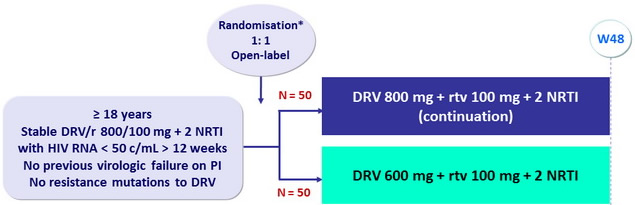
* Randomisation was stratified on HIV RNA (≤ or > 100,000 c/ mL ) prior to ART start
Objective
- Primary Endpoint : proportion with treatment success at W48 (ITT analysis)
- Assuming 90% efficacy at W48, sample size of 100 provide 80% power to detect a minimum difference of 15% in efficacy
- Other endpoints : observed analysis of virologic efficacy, PK substudy, cost-efficacy analysis
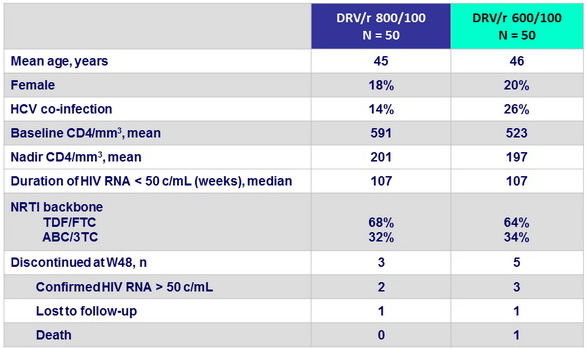
Baseline characteristics and disposition
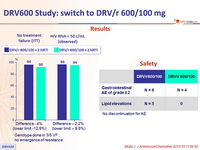
Results
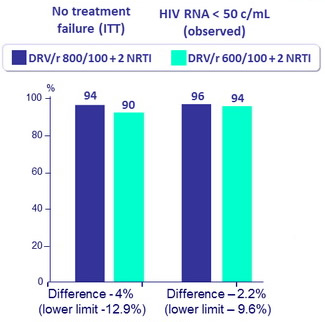
Genotype done in 3/5 VF : no emergence of resistance
Safety
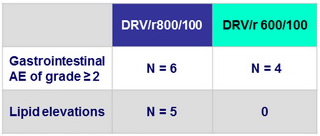
No discontinuation for AE
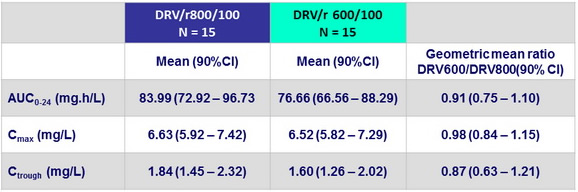
Phamacokinetics
- Mean DRV Ctrough
- 2.21 ± 1.44 mg/ dL for DRV/r 800/100 vs
- 2.19 ± 1.50 mg/ dL for DRV/r 600/100 (p = 0.94)
- No significant difference in AUC nor other PK parameters between the 2 groups
Full PK analysis