Molina JM, IAS 2019, Abs. LBPED46, Abs. WEAB0402LB
Type of ARV Trial
Phase 2 of new ARVs
» Islatravir (ISL)
Phase 2 of new ARVs
» Islatravir (ISL)
- Participants who initiated on ISL + DOR in combination with 3TC and switched to ISL + DOR had high efficacy at W48 as measured by proportion with HIV RNA < 50 c/mL similar to DOR/3TC/TDF
- No participant in any treatment group met criteria for resistance testing (All confirmed HIV RNA for protocol-defined virologic failure was < 80 c/mL)
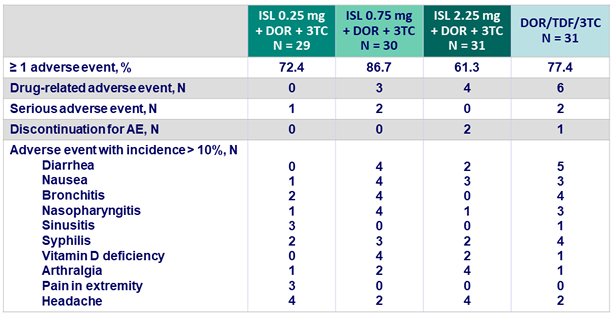
- ISL + DOR was generally well- tolerated
- Few drug-related adverse events (7.8% overall)
- Rate of discontinuation for adverse event was low (2.2%)
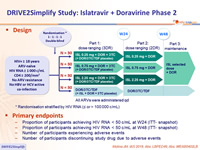
Design
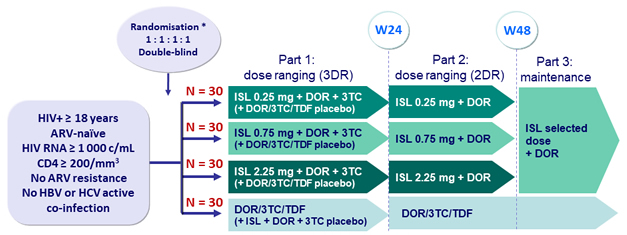
All ARVs were administered qd
* Randomisation stratified by HIV RNA (≤ or > 100 000 c/mL)
Primary endpoints
- Proportion of participants achieving HIV RNA < 50 c/mL at W24 (ITT- snapshot)Â
- Proportion of participants achieving HIV RNA < 50 c/mL at W48 (ITT- snapshot)Â
- Number of participants experiencing adverse events
- Number of participants discontinuing study drug due to adverse events
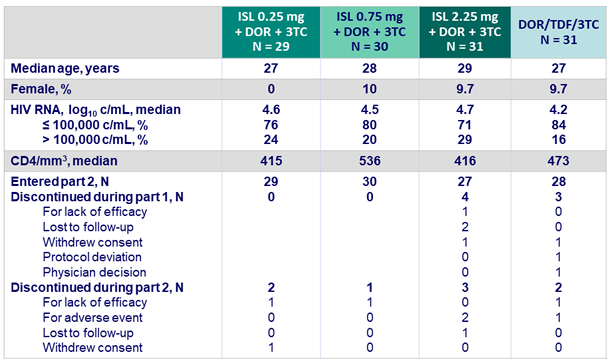
Baseline characteristics and patients disposition
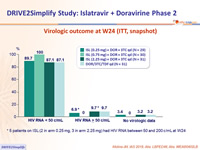
Virologic outcome at W24 (ITT, snapshot)
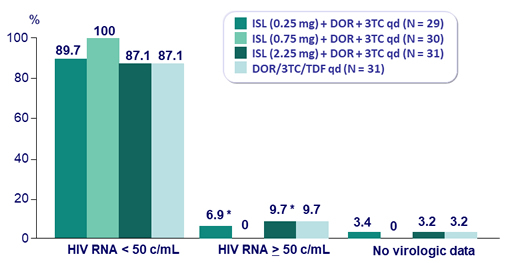
* 5 patients on ISL (2 in arm 0.25 mg, 3 in arm 2.25 mg) had HIV RNA between 50 and 200 c/mL at W24
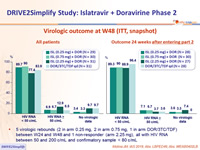
Virologic outcome at W48 (ITT, snapshot)
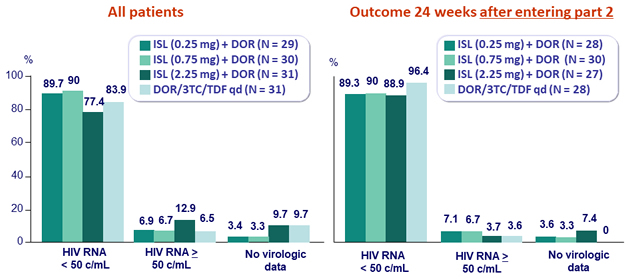
- 5 virologic rebounds (2 in arm 0.25 mg, 2 in arm 0.75 mg, 1 in arm DOR/3TC/TDF) between W24 and W48 and 1 non-responder (arm 2.25 mg), all with HIV RNA between 50 and 200 c/mL and confirmatory sample < 80 c/mL
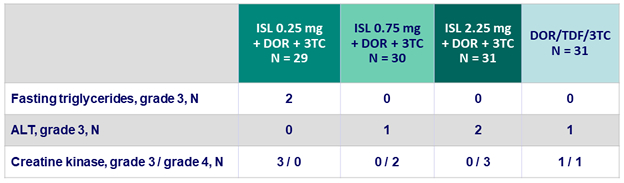
Adverse events
Grade 3 or 4 laboratory abnormalities