Johnson M. J Acquir Immune Defic Syndr. 2019 Apr 11. [Epub ahead of print]
Type of ARV Trial
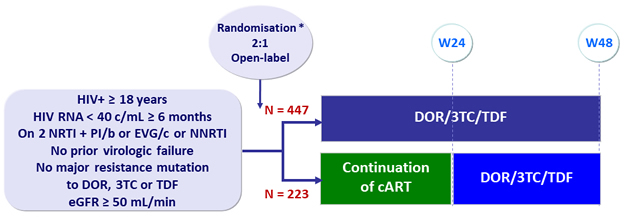
Switch studies in virologically suppressed patients
» Switch to DOR/3TC/TDF
Switch studies in virologically suppressed patients
» Switch to DOR/3TC/TDF
Drugs
TDF, 3TC, DOR
TDF, 3TC, DOR
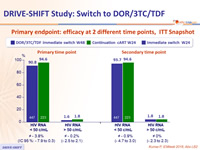
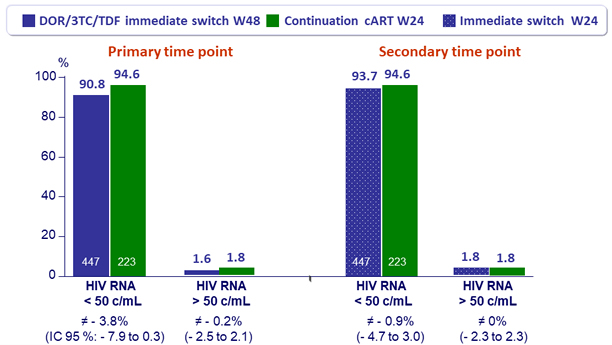
- Switching to DOR/3TC/TDF demonstrated non-inferior efficacy, at W24 and W48, compared to continuation of baseline cART through W24
- No emergence of resistance to DOR, 3TC or TDF
- Favorable safety profile
- Higher incidence of adverse events in participants who switched to DOR/3TC/TDF compared with those who continued their baseline regimen
- Superior lipid profile for LDL-cholesterol and non-HDL cholesterol of DOR/3TC/TDF compared to continuation of a boosted-PI regimen
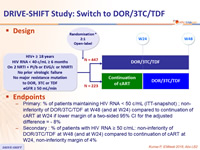
Design
Endpoints
- Primary: % of patients maintaining HIV RNA < 50 c/mL (ITT-snapshot) ; non-inferiority of DOR/3TC/TDF at W48 (and at W24) compared to continuation of cART at W24 if lower margin of a two-sided 95% CI for the adjusted difference = - 8%
- Secondary : % of patients with HIV RNA ≥ 50 c/mL: non-inferiority of DOR/3TC/TDF at W48 (and at W24) compared to continuation of cART at W24, non-inferiority margin of 4%
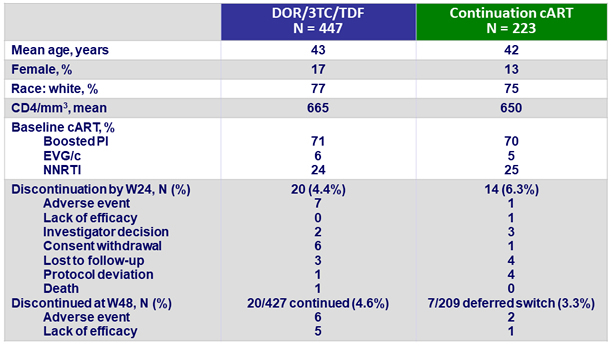
Baseline characteristics and patient disposition
Primary endpoint: efficacy at 2 different time points, ITT Snapshot
Drug resistance
- Resistance analysis population, DOR/3TC/TDF immediate and deferred switch
- Protocol-defined virologic failure, N = 7
- Discontinuation without protocol-defined virologic failure, N = 40
- No participant developed DOR or NRTI resistance
- All 24 participants with baseline NNRTI mutations (K103N, Y181C, G190A) remained suppressed
Adverse events, W24
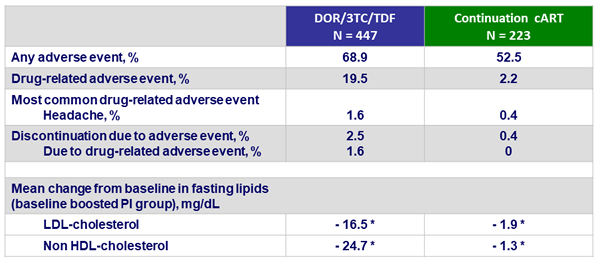
* p < 0,0001