Di Giambenedetto S. J Antimicrob Chemother 2017;72:1163-1171
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to PI/r + 3TC
» ATV/r + 3TC vs ATV/r + 2 NRTI
Switch studies in virologically suppressed patients
» Switch to PI/r + 3TC
» ATV/r + 3TC vs ATV/r + 2 NRTI
Drugs
ATV/r, 2 NRTI, 3TC
ATV/r, 2 NRTI, 3TC
- Simplification to ATV/r + 3TC in virologically suppressed patients on ATV/r + 2 NRTI is non-inferior and superior in a post-hoc analysis as compared with the continuation of the previous triple therapy at 48 weeks
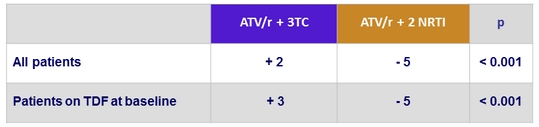
- A significant beneficial effect of ATV/r + 3TC in the evolution of eGFR was also observed, particularly in subjects discontinuing TDF
- In virologically suppressed patients on ATV/r + 2 NRTI who are not coinfected with hepatitis B virus, a switch to dual therapy with
ATV/r + 3TC may be considered
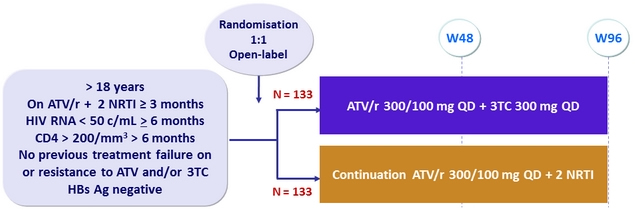
Design
Objective
- Primary Endpoint: proportion without treatment failure at W48
- Treatment failure: virological failure (the 1st of 2 consecutive HIV RNA levels > 50 c/mL or a single level > 1 000 c/mL), any treatment modification or discontinuation, loss to follow-up, consent withdrawal, progression to AIDS, or death for any cause
- Non-inferiority of ATV/r + 3TC (ITT-e and per protocol analyses) ; lower limit of the 95% CI for the difference = -12%, 80% power
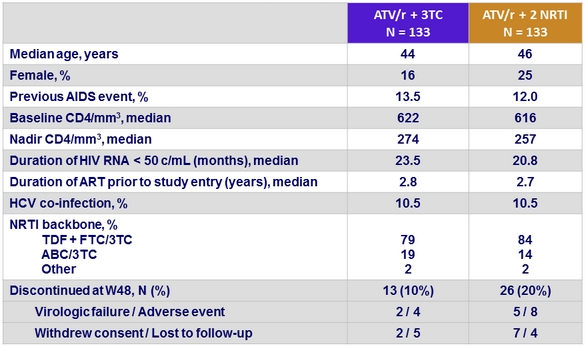
Baseline characteristics and disposition at W48
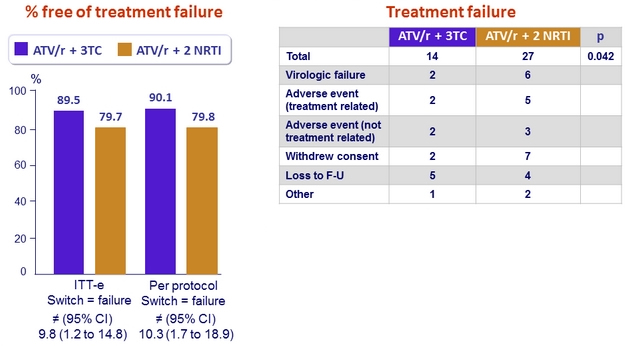
Efficacy Results (W48)
Virologic failure
- Genotype and quantification of ATV levels in 2/2 ATV/r + 3TC and 5/6 ATV/r + 2 NRTI
- No mutations on RT or PRO genes
- Undetectable ATV levels (< 0.05 mg/L): 1/2 ATV/r + 3TC and 3/5 ATV/r + 2 NRTI
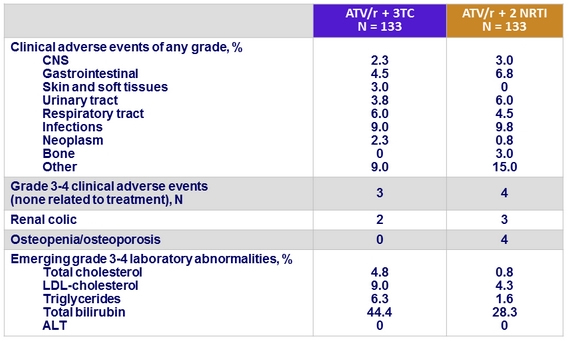
Adverse events
Mean change in eGFR (CKD-EPI formula) at W48, mL/min/1.73 m²
Lipids
- Significant increase in total cholesterol (p < 0.001), HDL-cholesterol (p = 0.001) and LDL- cholesterol (p = 0.047) in ATV/r + 3TC group, compared with ATV/r + 2 NRTI group
Adherence
- Self-reported adherence not different between study arms