Wohl D. PLoS One. 2014 May 13;9(5):e96187.
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to ATV or ATV-r
» ATV + ABC/3TC vs ATV/r + FTC/TDF
Switch studies in virologically suppressed patients
» Switch to ATV or ATV-r
» ATV + ABC/3TC vs ATV/r + FTC/TDF
Drugs
ATV/r, ATV, FTC/TDF, ABC/3TC, TDF, ABC, FTC, 3TC
ATV/r, ATV, FTC/TDF, ABC/3TC, TDF, ABC, FTC, 3TC
- Similar nature and rate of Grade 2-4 clinical adverse events
- Grade 2-4 adverse event leading to study withdrawal
- ATV + ABC/3TC, n = 8 (rash = 2, nausea = 3, vomiting = 2) ;
- ATV/r + TDF/FTC, n = 2
- Grade 3-4 laboratory abnormalities
- ATV + ABC/3TC : 19% (hyperbilirubinemia 6%)
- ATV/r + TDF/FTC : 36% (hyperbilirubinemia 29%) [p < 0.001 vs ATV + ABC/3TC]
- Significant improvements observed in bone biomarkers (BAP, PTH, CÂtelopeptide, and osteocalcin), renal urine b2 microglobulin/ creatinine ratio in the ATV + ABC/3TC group
Design :
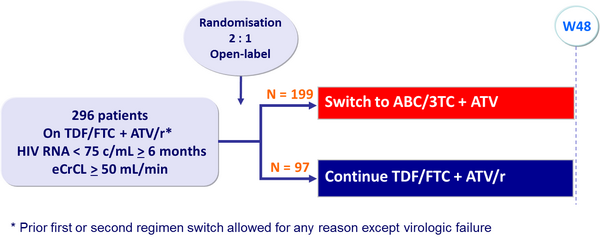
Primary Endpoints :
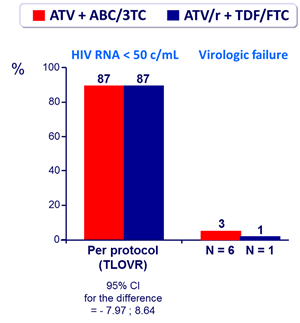
- Non inferiority in the proportion of patients with HIV RNA < 50 c/mL at W24 (TLOVR algorithm), lower limit of the 95% CI for the difference = - 12%
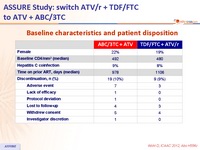
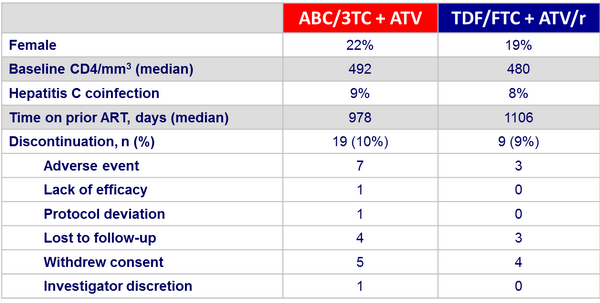
Baseline characteristics and patient disposition :
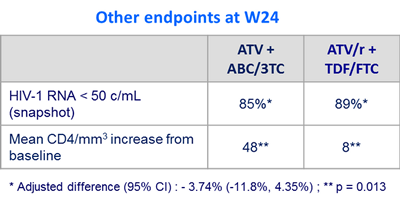
Outcome at week 24
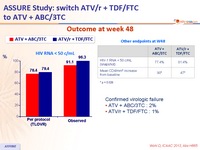
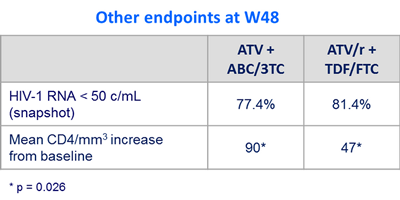
Outcome at week 48 :
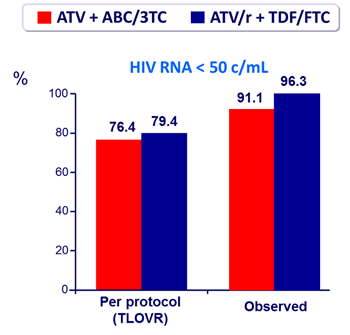
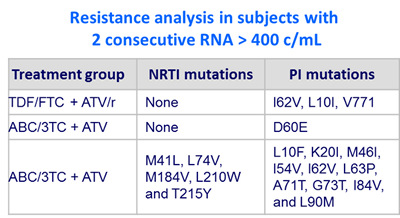
Confirmed virologic failure
- ATV + ABC/3TC : 2%
- ATV/r + TDF/FTC : 1%