Taiwo B. Clin Infect Dis. 2018 May 17;66(11):1794-1797
Type of ARV Trial
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG + 3TC
Switch studies in virologically suppressed patients
» Switch to DTG-containing regimen
» DTG + 3TC
Drugs
DTG, 2 NRTI, 3TC
DTG, 2 NRTI, 3TC
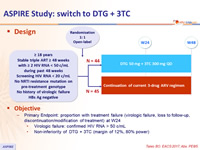
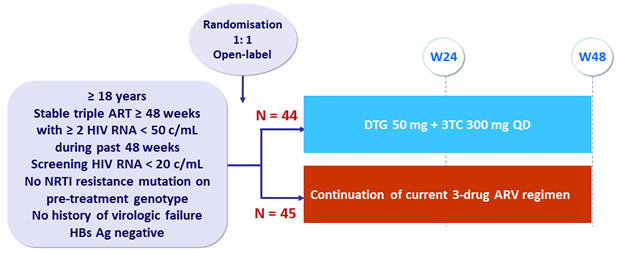
Design
Objective
- Primary Endpoint: proportion with treatment failure (virologic failure, loss to follow-up, discontinuation/modification of treatment) at W24
- Virologic failure: confirmed HIV RNA > 50 c/mL
- Non-inferiority of DTG + 3TC (margin of 12%, 80% power)
Baseline characteristics and disposition
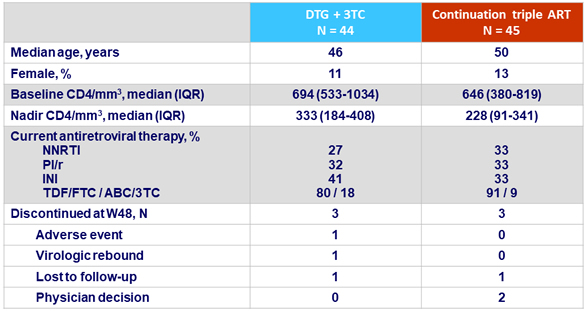
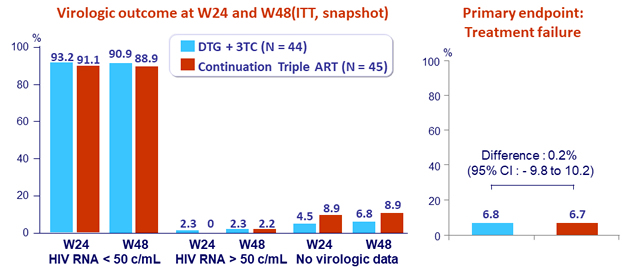
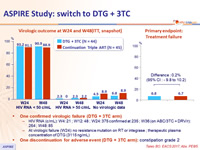
- One confirmed virologic failure (DTG + 3TC arm)
- HIV RNA (c/mL): W4: 21 ; W12: 48 ; W24: 375 confirmed at 235 ; W36 (on ABC/3TC + DRV/r): 264 ; W48: 85
- At virologic failure (W24): no resistance mutation on RT or integrase ; therapeutic plasma concentration of DTG (3115 ng/mL)
- One discontinuation for adverse event (DTG + 3TC arm): constipation grade 2